Antineoplastic Effect of Trastuzumab Deruxtecan in A Patient with A Previous Allogeneic Hematopoietic Stem Cell Transplantation
Abstract
Allogeneic
hematopoietic stem cell transplantation is a consolidated therapy for patients
with acute leukemia and for other diseases. Second neoplasms and relapses of
previously diagnosed malignancies are potential late complications of concern
for these patients, but current knowledge on the use of antineoplastic drugs in
this setting is scarce. Our case report describes an elderly female patient
with a previous history of allogeneic stem cell hematopoietic transplantation
that received long-term trastuzumab deruxtecan, a breakthrough antibody-drug
conjugate, for the treatment of advanced breast cancer with liver metastases.
We are unaware of previous reports on the use of this drug in patients with a
previous allogeneic transplant. Tolerance to the treatment has been excellent
and a complete response to the therapy sis maintained so far. As the starting
dose was the lowest registered for breast cancer, we review the published data
about starting dose reductions, but current information about this subject is
incomplete and further evidence is needed.
Keywords: Trastuzumab
deruxtecan; Breast cancer; Acute leukemia; Allogeneic stem cell transplantation
Introduction
The
potential appearance of second neoplasms are among the most significant late
complications of allogeneic hematopoietic stem cell or bone marrow
transplantation for leukemia and other diseases (ASCT)1. Current
knowledge about the systemic therapy of second neoplasms arising after an ASCT
is limited and there is a need to increase the information with reports and
studies about the activity and tolerance to new anticancer drug developments,
such as antibody drug conjugates, kinase inhibitors and immunotherapies in this
setting. We report herein the activity and tolerance to trastuzumab deruxtecan
(TDxd), a breakthrough antibody drug conjugate, in a patient with metastatic
breast cancer (BC) who had previously undergone an ASCT for acute leukemia.
Case Report
A
63-year woman (born in August 1954) was diagnosed with BC in Abril 2018.
Histopathology disclosed a phenotype Her2/neu grade 2 invasive ductal
carcinoma, with negative hormone receptors and overexpression of the human
epidermal growth factor receptor-2 (Her2/neu). Liver metastases were found on
radiologic staging. She started systemic therapy with the combination of
docetaxel plus pertuzumab and trastuzumab in May 2018. Chemotherapy with
docetaxel was maintained until December 2018, with imaging findings of complete
response at that time. Maintenance therapy with the antiHer2/neu monoclonal
antibodies pertuzumab and trastuzumab were continued until October 2020, when
the patient was admitted with a diagnosis of mixed-phenotype acute leukemia. BC
restaging showed a persisting complete response. Therapy for leukemia was
started according to the PETHEMA LAL 2019 protocol, with administration of the
FLAG-IDA scheme (fludarabine, cytarabine, granulocyte colony-stimulating
factor, idarubicin), with complete response. In March 2021 the patient
underwent reduced-intensity allogeneic stem cell transplantation from an
HLA-matched sister. Fludarabine plus melphalan were used for conditioning.
Prophylaxis of graft versus host disease (GVHD) was done with tacrolimus plus
methotrexate. A complete response of acute leukemia has been maintained, with
negative minimal residual disease. Ongoing controls have found a complete
chimerism and a mild chronic relapsing GVHD, with mainly ocular mucosal and
cutaneous involvement. Tacrolimus was stopped in September 2021.
Oncologic
evolution was uneventful until February 2023, when a relapse of BC in her right
breast was detected by positron emission tomography-computed tomography
(PET-CT), without findings of distant metastases. The patient underwent a
segmentectomy with axillary lymphadenectomy. Histopathology showed a grade 1
invasive carcinoma of 33 millimeters, with apocrine differentiation, 2/22
involved axillary nodes and a Her2/neu phenotype.
A
follow-up PET-CT detected in June 2023 the appearance of multiple liver
metastases, involving the segments IVa, III/IVb, V and VI (Figure 1).
Systemic therapy was started with TDxd. After taking into consideration the
context and the frailty of the patient, treatment was initiated with the lowest
registered dose of TDxd, corresponding to 3.2 mg/kg, aiming to a potential
increase of dose in case of good tolerance. There were signs of a nearly
complete response of liver metastases in the control PET-CT after three months
of therapy and of complete response in the control PET-CT studies done
afterwards (Figure 2). Tolerance to TDxd has been fair, with a mild
hematological toxicity and lack of pulmonary and cardiac complications. The
patient underwent surgery for cataracts in June 2025. Therapy with TDxd is
ongoing at the time of this report, with the 35th cycle started in September
2025. The dose of 3,2 mg/kg has been maintained, in consideration to her
excellent response and tolerance.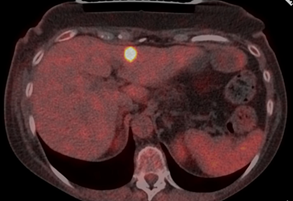
Figure 1: PET-CT imaging
at the start of therapy with TDxd, showing one of the liver metastases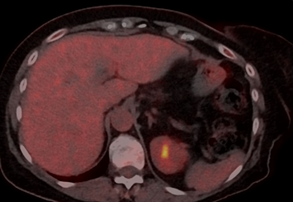
Figure 2: Follow-up PET-CT
during the treatment with TDxd, showing complete disappearance of the liver
lesion, the other liver lesions were also disappeared
Discussion
BC relapses after
ASCT for therapy-related myeloid neoplasms are uncommon. In a retrospective
study by the Chronic Malignancies Working party of the European Society for
Blood and Marrow Transplantation (EMBT), only 17 out of 252 patients with a
previous history of BC before ASCT had BC relapses, after a median follow-up of
20 months2.
Some reported
cases on the use of the antiHer2/neu antibody trastuzumab for BC therapy in
women or men after ASCT have been published in the context of localized3,4 or metastatic
disease5, in monotherapy or in
combination with paclitaxel, carboplatin or pertuzumab, without any specific
concern about the tolerance to these drugs in such context.
We are unaware of
any previous communication on the use of TDxd after an ASCT. There is one
published report on the use of TDxd in a BC patient with a previous history of
autologous (not allogeneic) bone marrow transplant for Hodgkin lymphoma6. Of note, this
patient also received a reduced dose of TDxd of 3,2 mg/kg, with good tolerance.
The pulmonary
toxicity of TDxd may be regarded as a potential concern for the use of this
drug after ASCT, but there were no significant clinical, imaging or functional
signs of lung complications with the long-term use of this conjugated antibody
in our patient. Authors from Slovenia have reported some worries about the use
of immune checkpoint inhibitors in this setting, not only for pulmonary risk,
but also for a potential aggravation of GVHD7. Furthermore, a
case of irinotecan-induced interstitial lung disease has been reported in a
patient with colorectal cancer and a previous history of ASCT8.
The use of a
reduced starting dose is obviously absent in the pivotal trials done for the
regulatory approval of TDxd and is uncommon in most observational reports
performed on this drug, not including those conducted in patients with severe
liver impairment. However, the Italian DE-REAL study concluded that the
real-world progressive-free and overall survivals were not affected by dose
reductions9. A retrospective
observational report from Florida described a 29% of patients with starting
dose reductions10. Data from the European
multicenter TREX-old registry reported a similar starting dose reduction in 30%
of the 158 patients included in this series of elderly BC women11. A higher
percentage of nearly or even more than 90% of patients with starting dose
reduction of TDxd have been described in two recently published Chinese
observational studies, but was influenced by economic reasons in at least one
of these reports12 and resulted in a
significantly negative impact of dose reduction on the efficacy of TDxd in the
other study13. On the other hand, another
observational study in an Asian population from Singapore reported no
significant impact on progression-free survival for a reduced dose of TDxd
resulting in <85% of relative dose intensity in a cohort of 87 patients,
with 47% of them starting with a reduced dose of this drug14.
Finally, the
large cohort of real-life data from the temporary use authorization program of
TDxd in France reported a starting reduced dose of 4,4 mg/kg for one woman with
BC and chronic myeloid leukemia among the 459 registered patients15. Data about the
treatment of the chronic myeloid leukemia is not available from this report,
but is described that the old age of that patient was another reason for
considering a starting dose reduction in this setting, as was also addressed
for our patient.
Conclusion
Our case report describes
the use of TDxd in an elderly patient with a previous ASCT that developed liver
metastases from BC. There has been an excellent response and tolerance to a
starting reduced dose of TDxd, that is ongoing during a long follow-up. Further
knowledge about dose management and other aspects of therapy in this setting
and for other patients with frailty is needed, as current data is incomplete
and with some conflicting results.
Acknowledgements
To all the health workers
implicated in the care of the patient.
Conflicts
of Interest
Jose Juan Illarramendi
received honorary fees from Daiichi-Sankyo.
Ethical Approval
Comité
de Etica de Investigación con Medicamentos de Navarra.
References
2. Nabergoj M, Mauff K, Beelen D, et al. Allogeneic hematopoietic cell transplantation in patients with therapy-related myeloid neoplasm after breast cancer: a study of the Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant 2022;57:1072-1078.
3. Alazhri J, Saclarides C, Avisar E. A rare complication resulting in a rare disease: radiation-induced male breast cancer. BMJ Case Rep 2016;2016:10.
4. Lowe T, Luu T, Shen J, et al. Male breast cancer 15 years after allogeneic hematopoietic cell transplantation including total body irradiation for recurrent acute lymphoblastic leukemia. Onkologie 2008; 31: 266-269.
5. Banna GL, Aversa SM, Crivellari G, et al. Administration of anti-HER2 antibody after nonmyeloablative allogeneic stem cell transplantation in metastatic breast cancer. Br J Cancer 2006;94:1550-1552.
6. Shazley O orr C. Hypercalcemia of Malignancy and Medication-Related Osteonecrosis of the Jaw Following Denosumab Discontinuation in Metastatic Breast Cancer: A Case Report. Cureus 2025;17(8):91082.
7. Pavlin T, Blatnik A, Šeruga B. Challenges in the management of operable triple-negative breast cancer in a survivor of the B-cell acute lymphoblastic leukemia: a case report. Front Oncol 2024;14:1404706.
8. Baba K, Matsubara Y, Hirata Y, Ota Y, Takahashi S, Boku N. Case report: Irinotecan-induced interstitial lung disease in an advanced colorectal cancer patient resurfacing decades after allogeneic bone marrow transplantation for aplastic anemia; a case report and narrative review of literature. Front Oncol 2023;13:1215789.
9. Botticelli A, Caputo R, Scagnoli S, et al. A. Real-World Outcomes of Trastuzumab Deruxtecan in Patients With HER2+ Metastatic Breast Cancer: The DE-REAL Study. Oncologist 2024;29:303-310.
10. Basta A, Lien K, Sun W, et al. A Real-World Single-Center Cohort Study on the Tolerability of Trastuzumab Deruxtecan for HER2+ Metastatic Breast Cancer. Oncology 2025:1-10.
11. Deleuze A, Nardin S, Klocker EV, et al. Real-world safety and effectiveness of T-DXd in older advanced breast cancer patients: Updated analysis in an expanded cohort from the TREX-old registry. ESMO Open 2025;10:104887.
12. Sang D, Su Y, Zhang Y, et al. Efficacy and safety of trastuzumab deruxtecan in Chinese patients with HER2-positive and HER2-low advanced breast cancer: a multicenter, observational, real-world study. Ther Adv Med Oncol 2025;17.
13. Wu S, Li J, Bian L, et al. Real-world efficacy and safety of trastuzumab deruxtecan in heavily pre-treated HER2-low metastatic breast cancer across distinct immunohistochemistry statuses. Transl Breast Cancer Res 2025;6:22.
14. Lee HY, Shih V, Chan JJ, et al. Evaluating the impact of relative dose intensity on efficacy of trastuzumab deruxtecan for metastatic breast cancer in the real-world clinical setting. Ann Acad Med Singap 2025;54:458-466.
15. Petit T, Hajjaji N, Antoine EC, et al. Trastuzumab deruxtecan in previously treated HER2-positive metastatic or unresectable breast cancer: Real-life data from the temporary use authorization program in France. Cancer Med 2024;13(9):7168.