Balloon Dilation in Septal Surgery: An Experimental Analysis of Unguided Balloon Motion
Abstract
Key
Points
• Unguided
nasal balloons displace with unintended motion at a critical angle of ~5
degrees.
• Closer
plate spacing increases balloon migration even at small angles, amplifying
instability.
• Results
support guided dilation systems to limit unintended forces on critical anatomy.
Keywords: Nasal surgery; Balloon dilation; Mechanical phenomena;
Equipment design; Endoscopy/methods
Introduction
Balloon
sinus dilation (BSD) represents a minimally invasive technique used in
sinonasal procedures to improve sinus patency without extensive mucosal
disruption1. The popularity of
BSD influenced the development of balloon-assisted septoplasty (BAS) techniques
to minimize dissection while providing access for FESS and other transnasal
procedures. One common FDA-approved septal mobilization balloon is the Relieva Tract
Balloon Dilation System, an unguided 16x40 mm balloon2.
Multiple
studies cite favorable safety outcomes, reflecting the growing popularity of
unguided balloons (UGB)3. Rare
complications of CSF leaks and orbital injuries are reported4-7. UGBs’ expansion between nonparallel
surfaces create uneven force vectors and unintended motion in the nasal cavity,
with the septum on one side and the lateral nasal wall and inferior turbinate
on the other. This asymmetry creates uneven force vectors, causing unintended
motion, a phenomenon consistent with complications in MAUDE analyses.
Despite
widespread use of UGBs, limited quantitative data exists regarding how surface
angle, spacing and friction influence balloon motion during dilation. Similar
mechanical concerns are described in Eustachian tube balloon dilation
procedures8. Guided balloons have
emerged, addressing these issues by improving control and reducing unwanted
motion9. However, to our
knowledge, this pattern of angle-dependent instability during nasal balloon
dilation has not been previously characterized in the published literature.
This study presents a controlled benchtop evaluation of UGB motion to determine
how these variables affect displacement and to identify the critical angle at
which stability is lost.
Methods
Testing
apparatus
The
experimental apparatus consisted of two 5 x 5-inch steel plates mounted on
threaded rods and spaced precisely with steel nuts and spacing washers. The
plates were lined with 3.25mm of 38A platinum-cure silicone to provide a
uniform elastic surface approximating soft tissue compliance.
Balloon
and inflation system
A
16 mm x 40 mm high-pressure non-compliant nylon balloon was used for all tests.
The balloon was inflated to a maximum pressure of 8 ATM using a 25cc
quick-latch inflation device capable of 30 ATM.
Experimental
procedure
The
plates were first aligned parallel, then adjusted to create varying surface
angles by inserting spacer washers at the upper bolts. The inferior plate
distance remained constant. The balloon was positioned in the lower portion of
the silicone-lined space and inflated initially to 1.5 ATM using manual plunger
action, then to the maximum recommended pressure using the threaded piston
screw mechanism.
Two
dry and lubricated surface conditions were tested with a water-based surgical
lubricant to simulate mucosal conditions. Each configuration was tested in five
consecutive trials (total=65 trials). Angles and distances were measured before
and after inflation using digital calipers.
High-resolution
video documentation was used to record balloon displacement and motion
patterns.
Results
Unguided
balloon displacement began when the surface angle reached approximately 5° from
parallel, with motion increasing as the angle widened. Angles were
progressively widened from 0 to 6.1, with a median value of 5.05° [4.7-5.35].
No movement was observed below 2°. At 5°- 6°, displacement ranged from 1.5 to
13 mm, with initial plate spacing of 8 mm. Consistent motion occurred at angles
of 5° or greater. Lubrication increased travel distance but not occurrence.
Median displacement was 7.15 [3.54-10.51] and 7.75 [4.0-10.381] for
non-lubricated and lubricated, respectively. At a 3.7°-angle, when plate
spacing was reduced to 6.94 mm, 3 mm and 2.51 mm, balloon movement increased. A
nonparametric Wilcoxon analysis confirmed a statistically significant
difference (Wstat =0, Wcritical =5 [~p<0.01]). (Figure 1) summarizes
the displacement trends with respect to angle with each point representing the
average of 5 trials, for a total of 65 trials.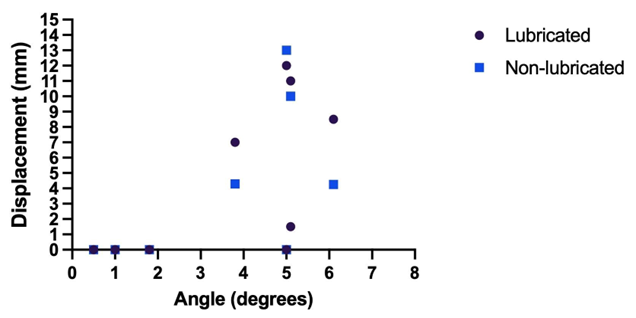
Figure
1: The displacement trends with respect to angle with each point
representing the average of 5 trials, for a total of 65 trials
Discussion
This
study establishes a framework for understanding UGB mechanics in sinonasal
procedures. Our findings revealed a consistent threshold of approximately 5° of
angular divergence between opposing surfaces, at which a high-pressure nasal
balloon transitioned from static to dynamic behavior. Beyond this threshold,
the balloon consistently migrates towards the wider gap, regardless of
lubrication conditions. Narrower plate spacing further amplified this effect,
suggesting that confined anatomical spaces like the nasal cavity may promote
UGB migration, even when tissue planes appear nearly parallel.
The
identification of a critical angle for motion has important implications for
device stability.
Displacement
became more pronounced at reduced spacing, indicating that the narrow septal
corridors encountered clinically can amplify balloon instability. Surface angle
and spacing were the primary determinants of displacement.
The
clinical relevance of these findings is underscored by the number of
complications reported with sinus UGB systems. Analysis of the FDA MAUDE
database has documented over 200 adverse events associated with balloon
sinuplasty devices (Acclarent, Entellus, Medtronic) including CSF leaks,
epistaxis, internal carotid artery dissections orbital swelling necessitating
emergency canthotomy and four periprocedural deaths. Of these complications,
17.8% were related to imprecise catheter movement and 39.6% to guide catheter
malfunction7. The predictable 5°
critical angle provides a mechanical explanation for these events, emphasizing
the need for device stability in confined anatomy.
These
results highlight the need for angle-aware, guided balloon systems. Devices
with rigid guiding elements, such as the ClearPathTM nasal balloon with a guide
spatula, redistribute forces medially and shield lateral structures, directly
addressing the angle-dependent instability revealed in this model10. In a recent study of endoscopic
eustachian tube balloon dilations, surgeons used image-guidance integration for
continuous catheter tracking and no major complications occurred9.
This
benchtop model simplifies complex in vivo anatomy. Rigid steel plates lined
with silicone do not fully replicate tissue compliance, surface irregularity or
mucosal friction. Additionally, the experimental pressures and spacing
distances, while clinically relevant, do not account for dynamic tissue
deformation or capillary adhesion. Cadaveric and computational models are
necessary to validate these findings in more realistic anatomical conditions.
Acknowledgements
None.
Funding
The authors self-funded this study.
Financial Disclosures
The authors have no financial disclosures.
Conflict of Interest
The authors have no conflicts of financial
disclosures or conflicts of interests to declare.
Reference
1. Fijałkowska-Ratajczak T, Kopeć
J, Leszczyńska M, Borucki Ł. Balloon sinuplasty in one-day surgery. Wideochir Inne Tech
Maloinwazyjne 2021;16(2):423-428.
2. MAUDE
Adverse Event Report: Acclarent, Inc. Relieva tract balloon dilation system,
16X40MM - 1PK; balloon 2025.
3. Liang X, Lan H, Liang X, et
al. Efficacy and safety of sinus balloon catheter dilation versus functional
endoscopic sinus surgery in the treatment of chronic sinusitis: A
meta-analysis. Medicine
(Baltimore) 2025;104(24):e42841.
4. Lofgren DH, Brandon B Knight,
Shermetaro CB. Contemporary Trends in Frontal Sinus Balloon Sinuplasty: A Pilot
Study. Spartan Med Res
J 2024;9(3):123407.
5. Chaaban MR, Rana N,
Baillargeon J, et al. Outcomes and Complications of Balloon and Conventional
Functional Endoscopic Sinus Surgery. Am
J Rhinol Allergy 2018;32(5):388-396.
6. Sayal NR, Keider E, Korkigian
S. Visualized ethmoid roof cerebrospinal fluid leak during frontal balloon
sinuplasty. Ear Nose
Throat J 2018;97(8):34-38.
7. Hur K, Ge M, Kim J, Ference
EH. Adverse Events Associated with Balloon Sinuplasty: A MAUDE Database
Analysis. Otolaryngol
Head Neck Surg 2020;162(1):137-141.
8. Chisolm PF, Hakimi AA, Maxwell
JH, Russo ME. Complications of eustachian tube balloon dilation: Manufacturer
and User Facility Device Experience (MAUDE) database analysis and literature
review. Laryngoscope
Investig Otolaryngol 2023;8(6):1507-1515.
9. Choi SW, Lee SH, Oh
SJ, Kong SK. Navigation-Assisted Balloon Eustachian Tuboplasty for Eustachian
Tube Dilatory Dysfunction. Clin
Exp Otorhinolaryngol 2020;13(4):389-395.
10. Brenner MJ, Shenson JA, Rose
AS, et al. New Medical Device and Therapeutic Approvals in Otolaryngology:
State of the Art Review 2020. OTO Open 2021;5(4).