Comprehensive Imaging Manifestations in Transfusion-Dependent Beta-Thalassemia Major with Secondary Diabetes Mellitus: A Case Report
Abstract
Background
Transfusion-dependent
beta-thalassemia major (TDT) is characterized by systemic iron overload
affecting multiple organ systems. Iron deposition patterns follow distinct
distributions, with reticuloendothelial deposition occurring primarily in the
liver, spleen, lymph nodes and bone marrow following multiple transfusions.
Secondary complications including diabetes mellitus develop as a consequence of
pancreatic iron accumulation and beta-cell dysfunction1,2.
Case
Presentation
We report a
15-year-old female with TDT presenting with fever and newly diagnosed diabetes
mellitus. Sequential imaging studies demonstrated the comprehensive multi-organ
manifestations of iron overload and its complications. Computed tomography
revealed multiple hyperdense lymph nodes with characteristic coarse peripheral
calcifications distributed throughout the porta hepatis, para-aortic and
peripancreatic regions, consistent with iron deposition in the
reticuloendothelial system1,3. The liver demonstrated significant enlargement with a beaver tail
variant of the left lobe, while cholelithiasis was present with calculi
measuring up to 7 mm. Post-splenectomy status was noted, which has been
associated with accelerated pancreatic iron deposition4. Chest
radiography showed rapid progression from normal lung fields to non-homogeneous
right-sided opacities over a 4-day interval. Ultrasonography revealed
hepatomegaly, pancreatic hyperechoic changes suggesting iron deposition and
significantly underdeveloped reproductive organs including an infantile uterus
and streak ovaries, indicating hypogonadotropic hypogonadism secondary to
iron-related hypothalamic-pituitary dysfunction2-4.
Discussion
This case
illustrates the reticuloendothelial pattern of iron deposition characteristic
of transfusion-related hemochromatosis, where iron accumulates predominantly in
Kupffer cells, splenic macrophages and lymph node reticuloendothelial cells3. The
development of secondary diabetes mellitus correlates with pancreatic iron
accumulation, which begins in early childhood and progressively affects
pancreatic beta-cell function5. The hyperdense lymphadenopathy observed on CT reflects iron-laden
reticuloendothelial cells within expanded cortical and medullary sinusoids, a
finding first described by Mitnick et al. in 19816. The concurrent endocrine
manifestations, including hypogonadism and diabetes, demonstrate the
multi-system impact of chronic iron toxicity beyond the classical hepatic and
cardiac complications1-3.
Conclusion
This case
demonstrates the comprehensive imaging spectrum of transfusion-dependent
thalassemia major, highlighting the reticuloendothelial pattern of iron
distribution and its associated complications. Early recognition of these
imaging findings is crucial for guiding intensive chelation therapy and
preventing irreversible organ damage, particularly in the pancreas and
endocrine system where iron-induced dysfunction can significantly impact
long-term patient outcomes.
Keywords: Beta-thalassemia major;
Iron overload; Reticuloendothelial deposition; Diabetes mellitus; Lymphadenopathy;
Hypogonadism; Comprehensive imaging
Introduction
Thalassemia
and structural hemoglobin variants are the most common monogenic disorders
worldwide. In India, the disease burden is significant, with an estimated
100,000 individuals affected by β-thalassemia syndromes and about 150,000 by
sickle cell disease. However, most patients do not receive adequate care and
curative treatment such as allogeneic stem cell transplantation remains beyond
the financial reach of most families7.
The
introduction of regular red cell transfusion therapy six decades ago changed
β-thalassemia majorly from a fatal disease of childhood into a manageable
chronic condition. Subsequent progress in preventing transfusion-related
infections and controlling iron overload has further improved outcomes,
enabling patients to achieve survival rates and quality of life that are now
close to normal8.
Survival in
transfusion-dependent β-thalassemia (TDT) has improved with advances in
transfusion protocols and oral iron chelation therapy. However, mortality
continues to remain high in India and other low- to middle-income countries. At
26.9 years of age, actuarial survival is only 50% with under-5 mortality being
7 times higher than in the general population. The most common cause of death
in these patients is infection9.
Radiological
evaluation plays a crucial role in monitoring disease progression and detecting
complications in thalassemia patients. Cross-sectional imaging modalities,
particularly computed tomography (CT) and ultrasonography, can demonstrate
characteristic findings of iron deposition organ dysfunction and associated
metabolic disturbances. We present a comprehensive case demonstrating the
spectrum of imaging manifestations in a teenage patient with
transfusion-dependent thalassemia major complicated by secondary diabetes
mellitus10-12.
Case
Presentation
Patient
demographics and clinical history
A
16-year-old female, Pranali Prakash Hailndgear, presented with persistent fever
for 10 days, body ache and recent onset of polyuria and polydipsia. She had a
well-documented history of beta-thalassemia major diagnosed in infancy,
requiring regular blood transfusions every 3-4 weeks. Splenectomy was performed
at age 8 years due to hypersplenism. The patient had been receiving iron
chelation therapy with deferasirox.
Growth
parameters consistently tracked at the 3rd percentile for age. Physical
examination revealed characteristic thalassemic facies, pallor and absence of
secondary sexual characteristics. Family history was significant for
thalassemia trait in both parents.
Laboratory findings
· Hematological: Severe microcytic hypochromic anemia (hemoglobin: 7.4 g/dL),
requiring transfusion.
· Metabolic: Random blood glucose 598 mg/dL, confirming diabetes mellitus.
· Iron studies: Markedly elevated serum ferritin (11,896 ng/mL), indicating severe
iron overload.
· Endocrine: Lower side of normal follicle-stimulating hormone levels (0.35
mIU/ml).
· Infectious markers: Hepatitis C virus serology positive, PCR negative.
Imaging Findings
Computed tomography of abdomen and
pelvis
Technical parameters: Contrast-enhanced study performed using 120 kVp, with oral and
intravenous contrast administration.
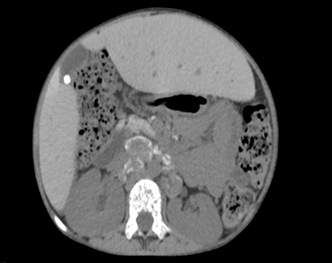
Key Findings: Contrast-enhanced CT of the abdomen and pelvis in this patient
revealed multiple enlarged lymph nodes with characteristic hyperdense coarse
peripheral calcifications distributed throughout the portal, left
gastro-hepatic, pre and para-aortic, aorto-caval and peripancreatic stations,
with the largest measuring 40.8 x 28.9 mm in the aorto-caval region, all
demonstrating uniform post-contrast enhancement (Figure 1).
The liver showed
enlargement of the left lobe extending to the left hypochondrium consistent
with a beaver tail variant (right lobe span 12.9 cm), with smooth contours and
no focal lesions. Notably the gallbladder was distended with a 6 mm hyperdense
calculus in the fundus.
The spleen was absent
consistent with prior splenectomy.
The pancreas, kidneys,
ureters and adrenals appeared normal with appropriate contrast enhancement.
The visualized axial
skeleton demonstrated coarsened trabeculae characteristic of thalassemic bone
changes, with no paravertebral masses identified (Figure 2).
No ascites, hydronephrosis
or other significant abnormalities were observed in the bowel loops, pelvis or
lower lung fields.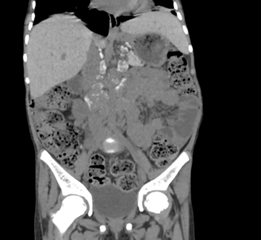
Figure 1: Coronal reformatted non contrast CT images after the administration
of neutral oral contrast showing multiple retroperitoneal lymph nodes
demonstrating characteristic hyperdense coarse peripheral calcification (as
noted by the arrows) in the left gastro-hepatic, pre- and para-aortic and
aorto-caval groups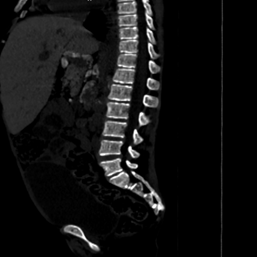
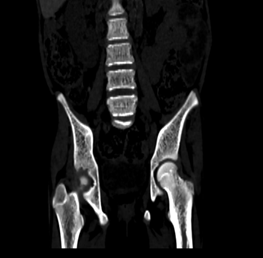
Fig 2(a) Fig 2(b)
Figure 2(a): Non
contrast CT abdomen axial image showing hepatomegaly with left lobe
enlargement, extending to the left hypochondrium (beaver tail variant), 2(b): Non contrast axial
image of the abdomen showing a distended gall bladder containing a 7 mm
hyperdense calculus (arrow)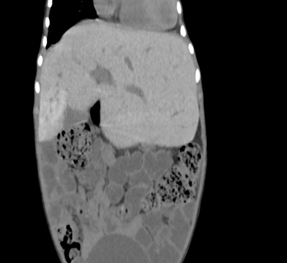
Figure
3(a,b): Bone window
CT images of the abdomen in the sagittal and coronal plane window showing
coarse trabeculations in the axial skeleton
Abdominal
ultrasonography
Abdominal
and pelvic ultrasonography in this patient demonstrated hepatomegaly with the
liver measuring 14.7 cm, with enlarged left lobe and normal echogenicity.
Cholelithiasis was consistently present, with gallstones measuring up to 6 mm
on follow-up, contained within a normally distended gallbladder with normal
wall thickness.
The pancreas
showed interval development of hyperechoic echotexture, potentially reflecting
iron deposition or early diabetic changes, correlating with the newly diagnosed
diabetes mellitus noted in the clinical history. Notably, the study
reproductive system abnormalities including an infantile uterus (corpus length
1.7 cm, AP diameter 0.3 cm; cervix length 1.7 cm, AP diameter 1.3 mm) and
bilateral streak ovaries (right: 0.7 x 0.1 cm, left: 1.0 x 0.1 cm), consistent
with hypogonadotropic hypogonadism secondary to iron overload affecting the
hypothalamic-pituitary-gonadal axis (Figure
3).
Both kidneys
maintained normal size, echogenicity and corticomedullary differentiation
throughout both examinations.
These
findings illustrate the multi-organ complications of thalassemia major,
including hepatic iron overload, cholelithiasis, pancreatic dysfunction and
endocrine failure manifesting as delayed puberty and diabetes mellitus.
Discussion
This case
exemplifies the comprehensive imaging spectrum encountered in advanced
transfusion-dependent beta-thalassemia major with secondary complications. The
radiological findings can be systematically categorized into several key areas
of organ involvement.
Iron
overload manifestations
The most
striking finding was the extensive retroperitoneal lymphadenopathy with
characteristic hyperdense rims on non-contrast CT imaging. This appearance is
pathognomonic of iron deposition (hemosiderosis) within lymphoid tissue, a
direct consequence of chronic transfusion therapy. The hyper density reflects
the paramagnetic properties of deposited iron, which appears hyperattenuating
on CT. The uniform post-contrast enhancement pattern distinguishes these nodes
from neoplastic processes, which typically demonstrate heterogeneous
enhancement patterns1.
Hepatomegaly
in this patient represents both extramedullary hematopoiesis and iron
deposition within hepatocytes and Kupffer cells. The preservation of smooth
hepatic contours without focal lesions suggests early-stage involvement without
significant fibrosis, though histological correlation would be definitive10.
Biliary complications
Cholelithiasis
is a well-recognized complication of chronic hemolytic disorders, including
thalassemia13. The formation of pigment stones results from increased bilirubin
production due to chronic hemolysis, compounded by post-splenectomy changes
that can alter red blood cell survival. The hyperdense appearance of the
calculus on CT suggests a high concentration of calcium bilirubinate14.
Endocrine dysfunction
The
ultrasonographic demonstration of infantile uterus and streak ovaries provides
direct imaging evidence of hypogonadotropic hypogonadism, a common endocrine
complication of transfusional iron overload. Iron deposition in the anterior
pituitary gland disrupts gonadotropin-releasing hormone production, leading to
delayed or absent puberty. This finding correlates with the patient's physical
examination and low FSH levels11,12.
Secondary
diabetes mellitus
While not
directly visualizable on imaging, the development of diabetes mellitus in this
patient reflects pancreatic iron deposition affecting beta-cell function.
Future imaging with MRI using T2* sequences could quantify pancreatic iron
content and monitor the efficacy of chelation therapy15.
Skeletal manifestations
The
coarsened trabecular pattern observed on CT represents the skeletal response to
chronic anemia and extramedullary hematopoiesis. Marrow expansion led to
cortical thinning and altered trabecular architecture, predisposing patients to
pathological fractures10,16.
Infectious
Complications
The
development of pulmonary opacities on chest radiography warrants consideration
of opportunistic infections, as patients with thalassemia major have increased
susceptibility due to iron overload-induced immune dysfunction and
post-splenectomy status17,18.
Clinical implications
This
comprehensive imaging assessment demonstrates several key clinical
implications:
· Monitoring protocol: Regular imaging surveillance is essential for detecting
complications before they become clinically apparent.
· Chelation efficacy: The extent of iron deposition visualized suggests suboptimal
chelation therapy efficacy, warranting dose adjustment or alternative chelating
agents.
· Multidisciplinary care: The imaging findings support the need for coordinated care
involving hematology, endocrinology, cardiology and radiology services.
· Risk stratification: The combination of severe iron overload and end-organ dysfunction
places this patient at high risk for cardiac complications, which should be
evaluated with cardiac MRI.
Differential considerations
While clinical history and
imaging findings are characteristic of trans fusional iron overload,
differential considerations for retroperitoneal lymphadenopathy in young
patients should include:
· Lymphoma (typically
heterogeneous enhancement)
· Metastatic disease (rare in
this age group)
· Inflammatory conditions
(usually associated with systemic symptoms)
The combination of
hyperdense lymph nodes, hepatomegaly, cholelithiasis and endocrine dysfunction
in a patient with known thalassemia major makes iron overload the most likely
diagnosis.
Key learning points
· Characteristic CT findings of iron overload
include hyperdense retroperitoneal lymph nodes with uniform post-contrast
enhancement, distinguishing them from malignant processes.
· Ultrasonography effectively demonstrates
endocrine complications through visualization of hypoplastic reproductive
organs, correlating with clinical hypogonadism.
· Cholelithiasis in young thalassemia
patients typically represents pigment stone formation due to chronic hemolysis
rather than cholesterol stones.
· Comprehensive imaging assessment
is essential for monitoring disease progression and guiding therapeutic
interventions in complex thalassemia cases.
· Early detection of complications
through imaging surveillance can improve long-term outcomes and quality of life
in transfusion-dependent patients.
Conclusion
This case demonstrates the
comprehensive spectrum of imaging manifestations in advanced
transfusion-dependent beta-thalassemia major. The combination of iron overload
findings, endocrine dysfunction, biliary complications and skeletal changes
illustrates the multisystem nature of this condition. Cross-sectional imaging,
particularly CT and ultrasonography, provides valuable insights into disease
progression and complications, guiding clinical management and monitoring
therapeutic efficacy.
Radiologists should be
familiar with these characteristic findings to facilitate early recognition and
appropriate management of complications in thalassemia patients. Regular
imaging surveillance remains essential for optimizing long-term outcomes in this
complex patient population.
Compliance
with Ethical Standards
Funding
No funding was received for
this study.
Conflict
of interest
The authors declare that
they have no conflicts of interest.
Ethical
approval
This case report was
conducted in accordance with institutional ethical guidelines.
Informed
consent
Written informed consent was
obtained from the patient's legal guardians for publication of this case report
and accompanying images.
References
2. Siegelman ES, Mitchell DG,
Rubin R, et al. Parenchymal versus reticuloendothelial iron overload in the
liver: distinction with MR imaging. Radiology 1991;179(2):361-366.
3. Matter RM, Allam KE,
Sadony AM. Gradient-echo magnetic resonance imaging study of pancreatic iron
overload in young Egyptian beta-thalassemia major patients and effect of
splenectomy. Diabetol Metab Syndr 2010;2(1):23.
4. Queiroz-Andrade M, Blasbalg R ortega CD, et al. MR
Imaging Findings of Iron Overload1. 2009;29(6):1575-1589.
5. Siegelman ES, Mitchell DG,
Rubin R, et al. Parenchymal versus reticuloendothelial iron overload in the
liver: distinction with MR imaging. Radiology 1991;179(2):361-366.
6. Mitnick DM, Heyman RE, Slep
AMS. Changes in relationship satisfaction across the transition to parenthood:
a meta-analysis in 1981. J Fam Psychol 2009 Dec;23(6):848-852.
7. Madan N, Sharma S, Sood S,
Colah R, Bhatia H. Frequency of β-thalassemia trait and other
hemoglobinopathies in northern and western India. Indian J Hum Genet
2010;16(1):16–25.
8. Lal A. Challenges in
chronic transfusion for patients with thalassemia. Hematology: the American
Society of Hematology Education Program 2020;2020(1):160.
9. Dhanya R, Sedai A, Ankita K, et al. Life
expectancy and risk factors for early death in patients with severe thalassemia
syndromes in South India. Blood Adv 2020;4(7):1448.
10. Suryadevara M, Mishra
GV, Shetty ND, et al. Radiological Features of Extramedullary Hematopoiesis in
a Young Male with Beta-Thalassemia: A Case Report. Cureus 2024;16(7):63945.
11. Chowdhury
R, Iktidar MA, Ahmed MN, et al. Prevalence of hypogonadism in
transfusion-dependent β-thalassemia patients of Bangladesh: investigating the
role of serum ferritin level as a diagnostic tool. Hematol Transfus Cell Ther
2023;45(3):350-357.
12. The role of
pituitary MRI in assessing endocrine dysfunction and growth defects in
thalassemia major | ECEESPE2025 | Joint Congress of the European Society for
Paediatric Endocrinology (ESPE) and the European Society of Endocrinology (ESE)
2025: Connecting Endocrinology Across the Life Course | Endocrine Abstracts
2025.
13. Maulana
MB, Fuadi MR ul. Clinical pathology aspect on diagnosis cholelithiasis in
β-Thalassemia patient: A case report. Annals of Med Surg 2022;81:104454.
14. Damayanti EL,
Gayatri P. Cholelithiasis Diagnosis and Management in Thalassemia. Archives of
Pediatric Gastroenterology, Hepatology and Nutrition 2024;3(2):15-25.
15. Taneera J, Mahgoub E, Qannita
R, et al. β-Thalassemia and Diabetes Mellitus: Current State and Future
Directions. Horm Metab Res 2024;56(4):272-278.
16. Colah
R, Italia K, Gorakshakar A. Burden of thalassemia in India: The road map for
control. Pediatric Hematology Oncology J 2017 Dec 1;2(4):79-84.
17. Shah
FT, Sayani F, Trompeter S, Drasar E, Piga A. Challenges of blood transfusions
in β-thalassemia. Blood Rev. 2019;37:100588.
18. Patterson S, Singleton
A, Branscomb J, Nsonwu V, Spratling R. Transfusion Complications in
Thalassemia: Patient Knowledge and Perspectives. Front Med (Lausanne)
2022;9:772886.