Estimation of Titanium and Aluminium in Peri-Implant Gingival Tissue. A Preliminary Study
Abstract
In
the course of history, multiple implant surface options have been utilized to
maximize contact between bone and dental implant. At present, in oral
implantology, Grade V alloy i.e., pure titanium (cp-Ti) or Grade IV alloy,
which is composed of 6% aluminum (Al) and 4% vanadium (V), are most commonly
used. Leaching of metal ions in the gingival tissue from the implant have
always been a concern. Corrosion products resulting from the degradation of the
dental implant surface due to biological fluids and infection may accumulate in
the body and lead to clinical consequences. The long-term presence of corrosion
reaction products and ongoing corrosion lead to fractures of the alloy-abutment
interface, abutment or implant body. The combination of stress, corrosion and
bacteria contribute to implant failure. The present study aims to assess the gingival tissues around the dental implant for the
levels of titanium and aluminium metal ions.
Keywords: Dental implants; Metal concentration;
Corrosion; Implant failure; Peri-implantitis
Abbreviations
Ti-
Titanium, Al- Aluminium, ICP-MS-Inductively coupled plasma Mass Spectrometry,
ICP-OES Inductively coupled plasma Optical Emission Spectrometry.
INTRODUCTION
Osseo-integrated dental implants are designed to be in direct
contact with bone, ensuring a secure and stable fit. However, in spite of
biocompatibility, various challenges like acidic environment, biofilm and
saliva, implants are exposed to the risk of leaching of metal ions from the
implant surfaces is heightened. Additionally, titanium particles are released
from metallic instruments utilized during the implant drilling stage, from
implant surfaces during placement and from the implant-abutment interface1. The presence of titanium (Ti) is the primary element found in
peri-implant tissue, along with aluminum (Al) and vanadium (V). These metals
are a result of the corrosion of titanium dental implant alloy2. The present study involves
estimation of titanium and aluminium metal ion in the peri-implant gingival
tissue following placement of Grade IV dental implants in stage
II/stage III and Grade B periodontitis patients.
Materials
AND Methods
The
patients were selected from Out-patient Department of Periodontology,
Krishnadevaraya college of Dental science and Hospital. Inclusion criteria: Patients
selected for the study were patients with systemically healthy gingiva with the
age above 18 years having Stage II/ Stage III and Grade B periodontitis,
patients willing for Grade IV bone level implants and with no history of dental
implants, willing for 2 implants placement and having good oral hygiene
practices. Exclusion criteria, the study included: Patient having implanted
metallic devices, diet /occupational/personal exposure to metallic particles, parafunctional
habits and patient taking pharmacological agents influencing metallic exposure.
Pre-implant
placement we had procured 8 samples for Ti and Al metal ion estimation.
Following intervention 8 samples were again collected after 3 months, prior to
healing cap placement. So 16 samples pertaining Ti metal ions and 16 samples
pertaining Al ions were collected for analysis. On the same day of sample
collection, procured samples were sent to “Raghavendra Spectro Metullurgical
Lab”- 4th phase Peenya industrial area, Bangalore for assessment of
titanium and aluminium metal ions. A total of 16 samples were collected and
assessed for titanium and aluminium metal ion concentration. Each patient was
assessed for 2 metal ion concentration, i.e., Titanium and Aluminium.
The
sample size for the present study was estimated using GPower software (latest
ver. 3.1.9.7; Heinrich-Heine-Universi-ta ̈t Du ̈sseldorf, Du ̈sseldorf,
Germany). The sample size estimation was performed at 5% alpha error (α =
0.05), with an effect size of 1.60 [Based on Cohen classification, considering
a larger effect size (d=1.60) to observe between 2 groups in terms of Titanium and
Aluminium elements in the gingival tissues]. The power of the study was set at 80%,
revealed that a minimum of 16 samples were necessary for the present study. So,
each study arm will comprise of 16 samples. P value was set at P< 0.05.
Sample Preparation: Gingival tissues
were extracted through tissue punch (Figure1) from the designated implant
site and were kept in ependroff tube with 10% formalin solution. Gingival
tissue samples (Figure 2) were collected and assessed (Figures 3-7)
for ion concentration before implant placement was considered as baseline
(Control). Tissue samples collected at 3 months considered as test samples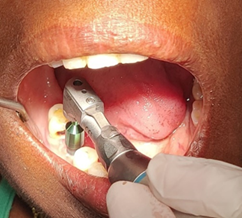
Figure
1:
Sampling before implant placement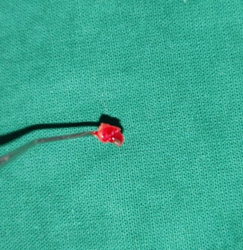
Figure
2:
Tissue sample obtained

Figure 3: Weight of sample is estimated

Figure 4: 5ml Nitric Acid is added

Figure 5: Complete Dissolution of sample
Figure 6: Pooling of samples

Figure 7: Avio 560 Max ICP optical emission spectrometer
RESULTS
In
the present study, the concentration of titanium and aluminium metal ions in
gingival tissue was assessed using ICP-OES. There was highly significant
difference for both the metal ions when comparison was done between before implant
placement gingival tissue and post 3 months peri implant tissue. (Tables 1
and 2) shows significant presence of titanium and aluminium metal ions
following placement of Grade IV dental implants.
Table1: Assessment of
difference in Titanium ions pre implant tissue and post implant gingival tissue.
|
|
pre implant placement |
post implant placement |
test statistic |
p value |
|
N |
9 |
9 |
-2.668c |
0.008** |
|
Mean |
5.7333 |
21.6278 |
|
|
|
Std. Deviation |
3.38231 |
20.01429 |
|
|
|
Median |
6 |
18 |
|
|
Table
2:
Assessment of difference in Aluminium ions pre implant tissue and post implant gingival
tissue
|
|
pre
implant placement |
post
implant placement |
test
statistic |
p
value |
|
N |
9 |
9 |
-2.668c |
0.008** |
|
Mean |
30.7778 |
63.2222 |
|
|
|
Std.
Deviation |
57.23368 |
78.0429 |
|
|
|
Median |
11 |
16 |
|
|
Discussion
Dental implant corrosion products may accumulate in human body and that there may be relationship between metal ion levels, peri-implantitis and implant failure2. This study was undertaken to investigate release of metal ions, in gingival tissue in vicinity of Grade IV dental implant. We have considered surrounding implant gingival tissue prior to implant placement as control group and implant tissue retrieved before healing cap placement at 3 months as test group for evaluation of titanium (Ti) and aluminium (Al) metal ions released following placement of dental implants.
Many studies have
been done to evaluate the metal ion concentration with dental implants in blood2, hair2, serum3 and saliva4. Because gingival tissue will
surround around immediate vicinity of dental implants, highest metal ion accumulation
is possible. Therefore, in our study we have considered gingival tissue
obtained from tissue punch before implant placement and post 3 months during
exposure of implant for healing cap placement.
Inductively
Coupled Plasma Optical Emission Spectrometry (ICP -OES) measurement was used to
detect titanium (Ti) and aluminium
(Al) metal ions. Similar to our study, Martin camean 2015, also have determined
the content of Aluminium, Cobalt, Chromium, Nickel, Titanium and Vanadium in
oro-mucosal cells of orthodontic patients with and without mini-implants using Inductively
Coupled Plasma Mass Spectrometry (ICP-MS)5. Contrarily, to their study our study showed there
is significant release of titanium
(Ti) and
aluminium (Al) levels in tissues, following 3 months after placement of Grade
IV implants when compared to the baseline values using Inductively Coupled
Plasma Optical Emission Spectrometry (ICP–OES).
Patients selected
for our study had stage II/stage III and grade B periodontitis, Inflammatory
condition have shown to influence implant corrosion rate in presence of
elevated inflammatory stress and hyperglycemia6. Galvanic corrosion and fretting
corrosion may release metal ions that may contribute to peri-implantitis and
implant failure7. Understanding
the metal ion release may provide us the information that may contribute to the
future consequences of implant placement. A low pH creates favorable
environment for aerobic bacteria for corrosion, contributing to microbial
corrosion7.
GCF and serum of
patients with periodontitis and healthy individuals were evaluated for levels
of trace elements like copper, zinc, selenium and chromium by Meenakshi B 2017
using Inductively Coupled Plasma Optical Emission Spectrometry (ICP–OES)3. Similarly in our study, we have
evaluated titanium (Ti) and aluminium
(Al) metal ions using Inductively Coupled Plasma Optical Emission Spectrometry.
Their study has concluded chromium levels were more in patients with
periodontitis than healthy. In our study we have compared prior to implant
placement and 3 months prior to prosthetic placement and concluded increased levels
of titanium (Ti) and aluminium
(Al) metal ions above the threshold level.
Altay B 2024, in
their study to measure the accumulation of titanium (Ti), aluminium (Al) and vanadium(V) in hair and blood
and secondarily aim to estimate their association between corrosion products
and fatigue outcome. They concluded that healthy dental implants, do not have a
significant impact on accumulation of titanium (Ti) ,aluminium (Al) and vanadium(V)in body
and have shown elevated Al levels in their group II (patients with
peri-implantitis) showing possibly due to infection influencing the corrosion
process2. Contrary to
their study, our study shows elevation of titanium (Ti) and aluminium (Al) levels at 3 months
following placement of dental implants in healthy gingival tissue. We conclude
that these factors may play a critical role in the existing oral environment to
tip towards peri-implantitis.
Lacey DC 2009, in
their study on effect of low dose metal particles, on monocyte/macrophages
survival concluded that their influence possibly can promote
monocyte/macrophages survival in vitro possibly via an endogenous mediator.
They directed, if this phenomenon occurs in vivo, increased number of
macrophages could contribute to local inflammatory reaction and osteolysis
critically showing implant failure8. Our preliminary study shows the release of titanium (Ti) and aluminium
(Al) metal ions in gingival tissues. The clinical scenario could detect the
future consequences depending upon the maintenance of oral health. Dissolution
of titanium from dental implants has an association to peri-implantitis9. There is also correlation of
effect of titanium (Ti) showing increased inflammatory cytokines from
surrounding host tissue cells10-12. These studies show the levels of metal ions having
immunological effects to titanium corrosion.
Our study clearly
demonstrates the level of titanium
(Ti) and
aluminium (Al) metal ion concentration in healthy gingival tissue immediately
before implant placement and post 3 months after implant placement in patients
with grade II/ III and grade B periodontitis. In our study many confounding
variables needed to be monitored which can have an influencing factor for metal
ions to tip towards peri-implantitis. Cumulative interceptive supportive
therapy (CIST) protocol needed to be followed for effective implant therapy.
Limitation of our study include, here we have included discarded tissue sample before
implant placement and after 3 months of post implant placement. We have
considered less (16) samples for evaluation and the tissue which is in vicinity
of implant for the duration of 3 months only. Metal ion concentration was
evaluated in punched out gingival tissue at post three months following dental
implant placement. Long term assessment with larger sample size may be
conclusive for the observed outcome.
Several
conclusions can be drawn from the present study. Firstly, our study
demonstrates that there is a definite release of titanium (Ti) and aluminium (Al) metal ion in the
gingival tissue following Grade IV dental implant placement compared to tissue prior
to implant placement. Long term assessment may necessitate a tissue biopsy for
evaluation. Importance of CIST protocol for implant patients’ needs to be
stressed for better clinical outcome.
Funding
Nil
Conflict
of interest
Nil
References
2. Altay, Berkan and Elif Çoban. Dental Implant Corrosion
Products May Accumulate in the Human Body. J Oral and Maxillofacial Surger:
official journal of the American Association of Oral and Maxillofacial Surgeons 2024;82:56-64.
7. Chaturvedi TP. An overview of the corrosion aspect of dental
implants (titanium and its alloys). Indian Journal Dental Res official
publication of Indian Society for Dental Research 2009;20:91-98.