Experiences on the Treatment of Locally Advanced Basal Cell Carcinoma with Vismodegib
Abstract
Basal
cell carcinoma (BCC) is the most common skin cancer. At early stages, various
topical treatments or light surgical procedures will give effective results.
However, when the commonly used treatments are not appropriated, the hedgehog
signaling inhibitor may be considered. We describe our experience on the
treatments of locally advanced BCCs with vismodegib.
Keywords:
Basal cell carcinoma; Gorlin Syndrome; Hedgehog signaling pathway inhibitor;
Vismodegib
BCC is often easily accessible and thus, topical treatments on superficial variants include cryotherapy with liquid nitrogen, photodynamic therapy (PDT), imiquimod or 5-fluorouracil. Nodular forms may be excised by a curette (curettage debulking) followed by cryotherapy or PDT or by surgery. Also, various lasers are used. Morpheaformic, infiltrative BCC and basosquamous carcinoma are targets for surgery1. In some cases, oncologic treatments by radiation therapy or cytostatic drugs may be used. In addition, various combination of the treatments may be applied. The newest treatment option are immunotherapies by anti-PD1- drugs2.
When surgery or other treatments are not applicable, Hedgehog signaling pathway inhibitors may be considered. Presently, there are two drugs in this group: vismodegib (Erivedge) 150 mg capsules and sonidegib (Odomzo) 200 mg capsules2. Both are very effective drugs, but are extremely teratogenic necessitating extreme cautions in handling of drug capsules.
Vismodegib and sonidegib demonstrate >99% binding to plasma proteins. Binding of vismodegib to proteins was concentration-dependent, whereas that of sonidegib was non-concentration-dependent. Vismodegib has a volume of distribution of 16-27 L, suggesting it is confined to the plasma and has limited tissue penetration. In contrast, sonidegib is more lipophilic and has a volume distribution of >9,000 L, indicating extensive distribution in tissues. Consequently, the concentration of sonidegib is 6 times higher in skin than in plasma. This might explain potential differences in efficacy and toxicity between these drugs. Vismodegib has an elimination half-life of 4-12 days achieving steady-state situation after 7-21 days. In contrast, sonidegib ‘s elimination half-life is longer 28-30 days achieving steady-state after 3-4 months3.
The efficacy of vismodegib (N=63) was reported in the BOLT and Erivance studies being 22.2% (complete clearance), 25.4% (partial clearance), 34,9% (stable disease), 12.7% (progressive disease) and 4.8% (unknown). These figures for sonidegib (N=66) were 21.2% (CR), 39.4% (PR), 30.3% (SD), 1.5% (PD) and 7.6% (unknown)3,4.
Vismodegib came available in Finland for patients in 2016 and sonidegib later in 2022. Sonidegib shares about the same efficacy, tolerability and adverse event profile as vismodegib. However, sonidegib has a better skin penetrance than vismodegib3. Financial monthly costs are at the same level.
Here we describe our experiences on the use of vismodegib for the treatment of locally advanced BCC, before sonidegib was available for our patients.
Case Reports
Case 1: A male born in
1951 started to develop multiple mainly superficial BCCs in the 1970s mainly in
the head area. He experienced numerous surgical operations in
Oto-Rhino-Laryngology and Surgery clinics and finally he was referred to
Dermatology Clinic in the 1990s. Gorlin Syndrome diagnosis was set. About 10 to
15 new BCCs developed during follow-up at about 1-to-2-month intervals. He was
treated by cryotherapy with liquid nitrogen and/or PDT.
In May 2013, an
investigational Phase-III Study by vismodegib was started until Oct 2016. At
the start he had 26 BCCs mainly in the head area, some in the upper trunk and
extremities, of which 4 target lesions were selected to be followed-up (Table
1). BCCs reduced in number and size very rapidly. In the following years he
could interrupt the medication for variable periods of 5 months to 1.5 years.
When 1 to 2 BCCs appeared, often excision and cryotherapy were performed. When
2 to 4 BCCs appeared, they were often excised with cryotherapy followed by
vismodegib for 2 to 3 months. When 14 BCCs were detected at a follow-up visit,
vismodegib was used for 6 months. PDT-treatment was used only once.
Table 1: A 62-year-old male with Gorlin syndrome treated with vismodegib during Phase-III study (Case 1)
|
Visit
day |
Total
number of BCCs |
Target
lesion 1 (mm) |
Target
lesion 2 (mm) |
Target
lesion 3 (mm) |
Target
lesion 4 (mm) |
|
8.5.2013 |
26 |
11 |
11 |
11 |
18 |
|
4.6.2013 |
19 |
0 |
7 |
9 |
0 |
|
2.7.2013 |
15 |
0 |
0 |
9.5 |
0 |
|
30.7.2013 |
6 |
0 |
0 |
2.5 |
0 |
|
27.8.2013 |
3 |
7 |
0 |
0 |
0 |
|
23.9.2013 |
0 |
0 |
0 |
0 |
0 |
|
23.10.2013 |
0 |
0 |
0 |
0 |
0 |
|
19.11.2013 |
0 |
0 |
0 |
0 |
0 |
|
17.12.2013
(br) |
0 |
0 |
0 |
0 |
0 |
|
14.1.2014
(br) |
0 |
0 |
0 |
0 |
0 |
|
11.2.2014 |
0 |
0 |
0 |
0 |
0 |
br = break in
vismodegib use started
Selected target
BCC lesions according to Study Protocol
Case 2: A male born in
1953 started to develop multiple mainly superficial BCCs in the 1970s typically
in the head area, but also in the upper trunk. He was the younger brother of
Case 1 and experienced numerous surgical operations in Oto-Rhino-Laryngology
and Surgery clinics. Finally, he was referred to Dermatology Clinic in the
1990s and Gorlin Syndrome diagnosis was established. About 10 to 15 new BCCs
developed during follow-up at about 1-to-2-month intervals. The treatments
included cryotherapy with liquid nitrogen and occasional PDTs. The biggest
superficial BCC with a size of 8x15 cm located below the left clavicle and was
treated in 6 visits by cryotherapy.
In May 2013, an investigational Phase-III Study by vismodegib was started. At the start he had 40 BCCs mainly in the head area, some in the upper trunk, of which 5 target lesions were selected to be followed-up (Table 2). BCCs reduced in number and size very rapidly. However, his head skin was full of scars due to multiple operations and some target lesions responded in variable manner. When new occasional lesions developed, vismodegib was used without interruptions.
Table 2: Basal cell
carcinoma treatment with vismodegib during Phase-III study of Case 2 (Gorlin
syndrome
|
Visit
day |
Total
number of BCCs |
Target
lesion 1 (mm) |
Target
lesion 2 (mm) |
Target
lesion 3 (mm) |
Target
lesion 4 (mm) |
Target
lesion 5 (mm) |
|
8.5.2013 |
40 |
33 |
10 |
13 |
12 |
17 |
|
4.6.2013 |
36 |
12 |
8 |
13 |
12 |
19 |
|
2.7.2013 |
32 |
15 |
8 |
17 |
14 |
16 |
|
30.7.2013 |
18 |
3.5 |
4 |
5 |
0 |
14 |
|
27.8.2013 |
10 |
6.5 |
4 |
9 |
0 |
0 |
|
23.9.2013 |
4 |
10 |
4 |
0 |
0 |
4 |
|
23.10.2013 |
1 |
0 |
3.5 |
0 |
0 |
0 |
|
19.11.2013 |
0 |
0 |
0 |
0 |
0 |
0 |
|
17.12.2013 |
0 |
0 |
0 |
0 |
0 |
0 |
|
14.1.2014 |
0 |
0 |
0 |
0 |
0 |
0 |
|
11.2.2014 |
1 |
0 |
0 |
0 |
0 |
0 |
Case 3: A male born in 1947 showed at the age of 46 in his left ear-cheek area a morpheaformic BCC that was operated numerous times with pure margins, but relapses were encountered. Also, radiation therapy was given in 2003. In 2007, petrosectomy and microvascular flap were performed and in preoperational pulmonary X-ray, a squamous cell carcinoma was detected and treated with cytostatic drugs. In 2013, a relapse BCC was detected infiltrating along the skull base being beyond surgery. It affected his jawbone and jaw joint causing difficulties in eating. Thus, vismodegib was initiated in March 2015 (Figure 1a) and the lesion shrank gradually during over 3 months (Figure 1b) and at 7 months it was clinically clear with the histopathology of scar. In addition, his yaw movement returned. Vismodegib was continued for a total of 1 year 3 months. At follow-up 6 months later (Figure 1c) in November 2016, a clinical remission was still noticed.
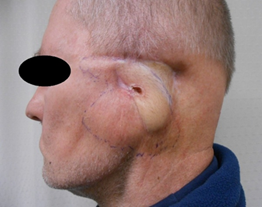
Figure 1a Figure 1b
Figure 1c
Figure 1: Treatment of morpheaformic basal cell carcinoma with vismodegib in a 68-year-old male (Case 3). Blue/black margins show the localization of tumor. At start in February-March 2015 (Figure 1a), after 3 months (Figure 1b), after 7 months being clinically and histopathologically clear. Vismodegib was continued for 1 year 3 months. At follow-up at 1 year 9 months later from start (Figure 1c), a clinical remission was noticed in November 2016.
Case 4: A 52-year-old
male had on the lateral right cheek in front of ear a nodular infiltrative BCC
growing gradually for 5 years with Staphylococcus aureus colonization. A
Computer Tomography revealed tumor tissue to be in close contact to mastoid
part of the occipital bone, dorsal part of temporal bone to lateral edge of
infratemporal fossa and to occipital and bone ear canal (Figure 2a). At
a multiprofessional meeting, surgery and radiation therapies were not
considered and vismodegib was started in September 2015 for 10 months at a
standard dose of 150 mg/day. The response started in a few weeks, at follow-up
a marked healing (Figure 2b) was noted and later after 5.5 months his
skin was clinically clear. Three years later, a relapse (Figure 2c) was
confirmed by Magnetic Resonance Image (MRI) and vismodegib was used for 3
months. At the follow-up of 5 months later the skin was clinically and by MRI
clear of disease. Two years later he was clinically in remission. The last
follow-up 5 years later in January 2015 (Figure 2d) still showed
remission, that is, over 9 years from the start of treatment.
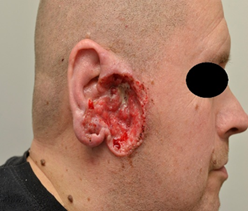
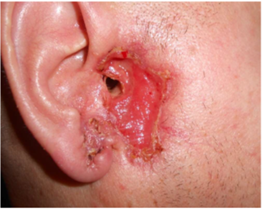
Figure 2a Figure 2
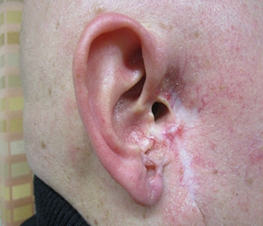
Figure 2c Figure 2d
Figure 2: Treatment of nodular infiltrative basal cell carcinoma with vismodegib in a 52-year-old male (Case 4) with Staphylococcus aureus colonization. At the start in February 2015 (Figure 2a) and after 2 months (Figure 2b) with a marked improvement. Vismodegib was used for 10 months. A relapse 3 years later (Figure 2c) was treated with vismodegib for 3 months. The last follow-up (Figure 3d) in January 2025 still showed clinical remission.
Case 5: A 72-year-old man
had suffered from wide superficial excoriations and wounds in his left leg for
3 years, despite adequate oral and topical treatments. In the bacterial
culture, Escheria coli was repeatedly found with an Extended Spectrum Beta
Lactamase (ESBL) resistency. Numerous skin biopsies revealed chronic wound and
regenerative tissue. However, BCC was finally confirmed in skin biopsy (Figure
3a). The patient participated in Phase-III Study by vismodegib and after 4
months a clear response was obtained (Figure 3b) and soon later the skin
was complete healed, without the need of leg amputation.
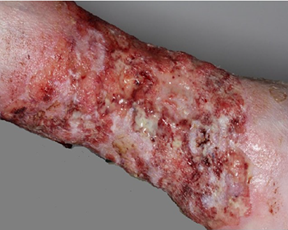
Figure 3a
Figure 3b
Figure 3: Treatment of
basal cell carcinoma with vismodegib in a 72-year-old male (Case 5) with
Escheria coli colonization. At the start (Figure
3a) and after 4 months (Figure
3b). The wide lesion healed without the need of leg
amputation. Courtesy from Dr Sari Pitkänen at the Department of Dermatology of
Helsinki University Central Hospital
Discussion
Hedgehog signaling pathway inhibitors are used only in
locally advanced BCC lesions when operational or other treatments are not
adequate. Both vismodegib and sonidegib are on the same level in efficacy, as
well as with adverse event profiles according to literature3,4. Most patients had
experienced typical adverse effects like hair loss, leg cramps and loss of
taste and thus, appetite. The patients with Gorlin syndrome felt it beneficial
to use oral magnesium citrate and magnesium containing topical cream products.
Vismodegib is very effective for the treatment of locally advanced BCCs. The patient 2 with Gorlin syndrome had experienced numerous surgical operations and thus, the facial skin was full of operational scars. Consequently, it Is possible that skin blood microcirculation was not sufficient for vismodegib to penetrate to the BCC tumor. This may explain, why some BCC lesions responded weakly or some new BCC tumors developed during vismodegib treatment, even though he used the drug continuously. However, individual properties are distinct, because the other Gorlin syndrome patient could have breaks in vismodegib treatments for several months, even up to 1.5 years.
The patient with morpheaformic BCC also responded well to vismodegib treatment, despite multiple wide surgeries previously.
The patient with right cheek-ear area BCC responded well without any heavy mutilating surgery and after a relapse he was treated again with another 3-month course with vismodegib. Of note, the patient was clinically clear as followed-up for 7years in January 2025. Cosmetically, the ear canal is now more visible but accepted by the patient.
Vismodegib treatment is usually not the 1st line treatment due to extreme teratogenicity, high costs (about 5,000 Eur/month) and to some extent the adverse events. It is an interesting question, when vismodegib should be used as the 1st line treatment.
The brothers with Gorlin syndrome responded well to vismodegib. However, it can be questioned what the efficacy would be without numerous previous surgical excisions and operations, when the skin blood vessel network is in a good condition delivering vismodegib to the BCC area. The results from the cases 4 and 5 (without earlier operations) might support the conclusion that vismodegib should be used earlier than recommended at the moment.
Another interesting question is, how long vismodegib treatment for each patient should go on without interruptions or if a break occurs, the length of such. We assume that it is neceessary to make an individual decision for each patient.
Ethical Approval
The
patients have given their consent for this case report.
Conflict of
Interests
Authors
declare no conflicts of interests.
References
1. Kwasniak, LA, Garcia-Zuazaga J. Review: Basal cell
carcinoma: evidence-based medicine and review of treatment modalities. Int J
Dermatol 2011;50:645-658.
2. Peris K,
Concetta Fargnoli M, Kaufmann R, et al. On behalf of
EADO, EDF, ESTRO, UEMS and EADV. European consensus-based interdisciplinary
guideline for diagnosis and treatment of basal cell carcinoma-update
2023. Eur J Cancer 2023;192:1-35.
3. Dummer R, Ascierto PA, Basset-Seguin N, et
al. Sonidegib
and vismodegib in the treatment of patients with locally advanced basal cell
carcinoma: a joint expert opinion. J Eur Acad Dermatol Venereol
2020;34:1944-1956.
4. Gutzmer R, Robert C,
Loquai C, Schadendorf D, Squittieri N, Arntz R, Martelli S, Dummer
R. Assessment of various efficacy outcomes using ERIVANCE-like criteria in
patients with locally advanced basal cell carcinoma receiving sonidegib:
results from a preplanned sensitivity analysis. BMC Cancer 2021;21(1):1-9.