Malignant Insulinoma: Case Report and Literature Review
Summary
Insulinoma is a neuroendocrine tumor of the pancreas
capable of secreting insulin. They are rare and the incidence varies according
to the center considered. Fasting hypoglycemia in a non-diabetic patient (blood
glucose below 55 mg/dl) is the most common clinical manifestation of insulinoma
and the presence of neuroglycopenic symptoms that may be preceded by autonomic
symptoms. The diagnosis of insulinoma requires inappropriately high levels of
insulin and C-peptide, associated with hypoglycemia. After the biochemical
diagnosis, the tumor must be located by imaging techniques such as ultrasound
and computed tomography of the abdomen. Malignant tumors have little or no
cellular pleomorphism, hyperchromasia or high mitotic activity, tend to have a
worse prognosis and the management of hypoglycemia, as well as the resolution
of associated metastases, is a challenge. The benefit of primary tumor
resection in patients with metastatic pancreatic neuroendocrine tumors is also
controversial.
Keywords: Hypoglycemia; Insulinoma; Neuroendocrine tumors
Introduction
Insulinoma has an incidence of approximately 4 cases per million inhabitants per year (1-3) and is frequently benign, single, sporadic and less than 20 mm1-5. Between 2-7% present as part of a Multiple Endocrine Neoplasia type 1 (MEN1) syndrome and up to 6% may present liver or lymph node metastases (malignant insulinoma)1-5.In insulinoma, a median age at diagnosis of 56 ± 18 years has been described and no differences between genders have been observed6.Its usual clinical presentation consists of the so-called Whipple Triad (characterized by the presence of symptoms: tremor, sweating, tachycardia; low blood glucose levels and relief of these symptoms by ingesting carbohydrates)5.Hypoglycaemia is mainly due to reduced hepatic glucose production, rather than increased glucose utilization7.
There are neuroglycopenic symptoms such as: confusion, visual changes and unusual behaviour and then sympathetic-adrenal symptoms: palpitations, diaphoresis and tremors. In addition, there could be hyperphagia, which can determine weight gain in these patients.
Diagnosis of insulinoma requires the demonstration of excessively high plasma insulin concentrations during an episode of spontaneous or provoked hypoglycaemia.
Once
the laboratory diagnosis has been made, the location of the tumour is required
and transabdominal ultrasound and computed tomography are preferred. When the
tumour is not located by initial imaging studies, additional options include
other non-invasive or invasive tests such as endoscopic ultrasound (endoscopic
ultrasound). This is a highly sensitive and specific procedure for the
localization of pancreatic endocrine tumours8.
Somatostatin receptor scintigraphy, on the other hand, is proposed for the detection
of insulinomas, when endoscopic ultrasound is negative9.
Other
non-invasive imaging tests include magnetic resonance imaging and
fluorine-18-L-dihydroxyphenylalanine positron emission tomography (18F-DOPA
PET) (10). Ga-68 gallium PET/CT (a somatostatin receptor-based imaging
modality) is an option when conventional imaging studies do not identify an
insulinoma. It is capable of identifying most of these tumours and can be
considered as a complementary imaging study when all imaging studies are
negative and when surgical treatment is planned11.
The
World Health Organization established a classification of malignant
gastroenteropancreatic neuroendocrine tumours based on a scheme of criteria
such as tumour size < 2 cm versus > 2 cm and presence of metastasis;
grade (mitotic rate, perineural and lymph vascular invasion, proliferative
index Ki-67)12.
Another
classification scheme estimates malignant potential using accurate prognostic
information by combining tumour size and metastases with simple classification
information based on necrosis and mitotic rate13.
Treatment
of sporadic insulinomas is surgical, but when it is more than one lesion, it is
debatable because it requires very extensive procedures with endocrine and
exocrine pancreatic functional loss.
The
liver and regional lymph nodes are the most common sites of metastatic disease.
In these cases, surgical treatment, hepatic artery embolization,
radiofrequency, cryoablation or even systemic therapy are considered14.
Medical
treatment to control hypoglycaemia is necessary in patients who are not subject
to surgery or in those who have unresectable metastatic disease or to control
recurrent hypoglycaemia while waiting for surgery14.
Diazoxide is used in the first line, but
octreotide or lanreotide because they are somatostatin analogues and as other
options verapamil or phenytoin can be used.
If
postoperative remission is achieved, the probability of recurrence of
insulinoma is low (<10%), but survival is worse in older patients and in
those with metastatic disease15.
Clinical
Case
A 33-year-old man with a history of psychiatric pathology treated with risperidone since childhood. In the previous three years, he presented multiple episodes of symptomatic hypoglycaemia that required hospital admission and management with continuous infusion of glucose serum, with blood glucose values that reached 35 mg/dl, seizures and loss of consciousness, which require management in intensive care. During the evaluation, a computed tomography of the abdomen was requested, which showed 2 pancreatic lesions, one in the body of 28 mm and another in the uncinate process of 42 mm. Additionally, multiple focal lesions were found in the liver, the largest of which was 32 mm (Figure 1). Likewise, venous glycemia of 37 mg/dl, insulin of 137 (normal range 2 to 12 μU/mL) and increased C-peptide of 8.27 (normal range 1.1 to 4.4 ng/ml) were found, which allowed a diagnosis of malignant insulinoma with liver metastases. A PET-CT scan with 18F-NOTATOC confirmed that pancreatic and hepatic lesions expressed somatostatin receptors (Figure 2a and 2b).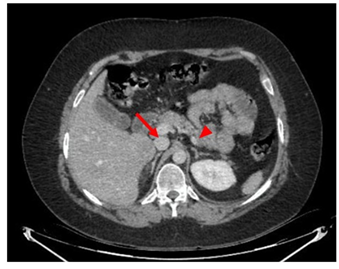
Figure 1: Axial computed tomography scan. Insulinoma is observed on the right in the uncinate process (arrow) and on the left (arrowhead)
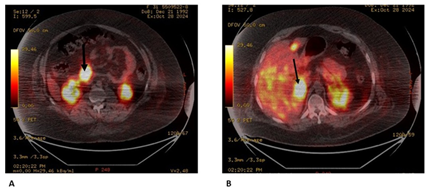
Figure
2:
PET with 18F-NOTA-OCT
A: insulinoma
uptake is observed (arrow);
B: uptake of
multiple liver metastases and the largest one (image on the right)
Since
the disease was in an advanced stage, surgical intervention was ruled out and
other treatments were chosen in parallel. In May 2022, two trans arterial
embolization’s were performed in which the lesion in the head of the pancreas
and a couple of liver lesions were eliminated, although the lesion in the body
of the pancreas and other hepatic lesions persisted. As pharmacological
treatment (May-June 2022), immediate-release octreotide (400 mcg c/8 hours) and
diazoxide (200 mcg c/8hs) were initiated.
Additionally,
a dose of lutetium was administered, with a good therapeutic response achieving
glycemia, between 100-200 mg/dl without the need for glucose serum and
significant reductions in insulinemia values: 12.8 μU/mL and C-peptide: 2.80
ng/ml. Under these conditions, he is discharged, with evolutionary control.
During follow-up, he received 3 additional doses of Lutetium: (August 2022,
November 2022 and January 2023). However, she did not maintain follow-up with
endocrinology and received diazoxide only for the first month, opting to
maintain only extended-release octreotide for 28 days. In October 2024 he was
admitted to hospital for episodes of symptomatic severe hypoglycaemia of up to
20 mg/dl. It was necessary to use a continuous infusion pump of 30% glucose
serum for stabilization. Given the persistence of hypoglycaemia, the use of
glucocorticoids was attempted to promote hepatic gluconeogenesis and reduce
hyperinsulinemia. However, this treatment was not effective and the infusion of
glucose serum was maintained. A new 18F-NOTE-OCT PET scan was performed, which
showed the same hyper uptake lesions in the pancreas and liver, but larger than
in the previous study.
In
addition, he presented intense bone pain that limited his ambulation, so a
pelvic MRI was requested that confirmed osteonecrosis of both femur heads.
Biochemical
analyses showed calcemia of 10.5 mg/dL (normal range of 8.3 - 10.3 mg/dL), PTH
of 243 pg/mL (normal range 15 - 65 pg/mL), with vitamin D < 3 ng/mL (normal
range 30-120 ng/mL), phosphate, azoemia and normal blood creatinine.
Urine/24-hour calciuria was also normal. With the approach of primary
hyperparathyroidism, a neck ultrasound was requested, which showed a
well-defined hypoechogenic solid nodular lesion, without vascularization of 9 x
7 x 7 mm, topographer in the left lower posterior thyroid sector. Parathyroid
scintigraphy could not be performed.
During
hospitalization, an attempt was made to embolize the lesions, but it was not
possible to locate the nutritious arteries of the tumours. Lutetium was
requested, but the tracer radio was not accessed. Despite the glucose serum and
diazoxide, the patient presented seizures that culminated in his death.
Discussion
The presence of
Whipple's triad, as in this case, supports the presence of pathological
hypoglycaemia. Adequate questioning and physical examination, with the
complementary laboratory, determine the aetiology of hypoglycaemia in a
non-diabetic patient. In people without underlying diseases, who do not take
drugs that cause hypoglycaemia like this patient, the most likely aetiologies
of hypoglycaemia are endogenous hyperinsulinism or factitious hypoglycaemia16.
The onset of
hypoglycaemic symptoms usually occurs when blood glucose levels drop below 55
mg/dL, although the specific threshold varies between individuals and over
time. The counterregulatory response (such as the release of glucagon and
epinephrine) may appear with glycaemic levels approximately 10 mg/dl higher,
before the onset of hypoglycaemic symptoms. In addition, glycaemic thresholds
for these counterregulatory responses may be higher in patients with insulinoma17.
Although fasting hypoglycaemia is the most
common presentation of insulinoma18,
postprandial hypoglycaemia may be a concurrent or even the only manifestation
of hypoglycaemia in some patients, although the proportion is low19.
Up to 20% of patients
with insulinoma receive a misdiagnosis of a neurological or psychiatric
disorder before the insulinoma is recognized20,21.
We are left with the
doubt in this case, treated for psychiatric pathology with episodes suggestive
of hypoglycaemia of three years of evolution, which were not previously studied
if they were not interpreted as part of his psychiatric illness of years of
evolution and therefore the diagnosis of hypoglycaemia is delayed.
There is a case series
report with insulinoma, which showed that approximately 77% presented autonomic
symptoms and 96% neuroglycopenic symptoms; ≥80% presented confusion or abnormal
behaviour, 50% lost consciousness or presented amnesia of the event and between
12 and 19% presented grand mal seizures22,23.
The presence of
recurrent neuroglycopenic symptoms, as in this case, warrants supervised
testing to detect insulin-mediated causes of hypoglycaemia22,23.
In the case of the
report, the patient had neuroglycopenic symptoms and a convulsive state of
great malice that requires admission to intensive care. And insulin and
C-peptide levels were inappropriately elevated for plasma glycemia, raising the
diagnosis of “hyperinsulinism hypoglycaemia” or hypoglycaemia with endogenous
hyperinsulinism. Once the biochemical diagnosis has been established, the
tumour causing insulin secretion must be located. Insulinomas can be single or
multiple, localized or metastatic.
Although most are
solitary tumours, approximately 10% of patients have multiple intrapancreatic
tumours14,24.
The vast majority
(≥90%) of insulinomas remain localized in the pancreas24,25.
Tumours are usually
small, with an average size of about 1.5 cm and can sometimes be difficult to
locate by preoperative imaging26.
In this case there were two pancreatic lesions but the size was just over 4 cm
the largest and that can speak of evolution time because he has been with
episodes of hypoglycaemia or tumour aggressiveness for three years due to a
potential for rapid growth.
The pathologic
appearance alone cannot determine whether a tumour is likely to be indolent and
confined to the pancreas, as opposed to aggressive and likely to metastasize.
Therefore, extension outside the pancreas is used as a marker of aggressive
clinical course and risk of future recurrence. A higher prevalence of MEN1 is
suggested among patients with multiple tumours (25%) and metastatic disease
(13%), compared to a prevalence of 7.6% in the general population (14). Others
report that up to 5-10% of insulinomas are associated with MEN-127, so it is essential to rule it out in
these patients. In our case, the presence of mild hypercalcemia and elevated
PTH suggests a possible diagnosis of primary hyperparathyroidism (PPH).
Although to confirm this, it is essential that vitamin D is in a range of
sufficiency, since its deficiency can raise PTH levels secondarily. But
hyperparathyroidism secondary to vitamin D deficiency usually does not cause an
increase in PTH well above 100 pg/ml, in this case it is much more. A finding
that reinforces the suspicion of PPH is the lesion described in the neck
ultrasound suggestive of parathyroid adenoma. In addition to osteonecrosis of
the femur, it could be a possible consequence of PPH28.
As stated above, the
ideal for the localization of the insulinoma is ultrasound and computed
tomography. In our case, the CT scan found the insulinoma and the PET scan was
added as a complementary study.
The treatment of choice
is surgery, which achieves cure in up to 90% of patients with benign
insulinomas4,5. In the case of
malignant insulinomas, surgery of the primary tumour increases survival between
12 and 28 months and allows better management of symptoms, although it is not
curative5.
Other therapeutic
strategies include invasive procedures such as chemical or radiofrequency
ablation or embolization of nutritive arteries, but there are also
pharmacological therapies for symptomatic control such as glucose serum,
diazoxide, somatostatin analogues, therapy with peptide receptor radionuclides
(lutetium) or chemotherapy4,5.
Medical treatment
includes fractionation of the diet, somatostatin analogues, diazoxide and
lutetium cone was raised in this case; but other drugs such as everolimus,
sunitinib could also be used when the previous ones did not work.
Somatostatin analogues
have been shown to not only improve symptoms and reduce tumour size in
well-differentiated tumours, but also stabilize the progression of liver
metastases in some cases.
In patients with
somatostatin receptor-positive pancreatic neuroendocrine tumours, such as in
this patient, if the disease is of low volume, initial treatment with a
long-acting somatostatin analogue such as octreotide and lanreotide is
suggested29.
In those with
symptomatic hepatic-predominantly low-volume disease, hepatic arterial
embolization, chemoembolization or radioembolization may be used.
In our case, the
patient had two pancreatic NETs and positive for somatostatin receptors
demonstrated with PET, but of high volume, so the initial treatment options
that can achieve a reduction in the disease burden include treatment with
radionuclides of peptide receptors with positive somatostatin receptors, plus
liver-directed therapy or capecitabine plus temozolomide that he did not
receive. Since they had a PET scan that showed radiotracer uptake, they may
benefit from treatment with these radiopharmaceuticals.
Treatment with
long-acting somatostatin analogues may retard tumour growth in patients with
locally advanced or well-differentiated somatostatin receptor-positive
pancreatic NETs and for that reason it was used in this case30.
In patients with
locally advanced, unresectable or metastatic pancreatic NETs that are positive
for somatostatin receptors, initial therapy with lutetium Lu-177 dotatate in
combination with octreotide or lanreotide improves tumour response compared to
high-dose octreotide31. For small
(< 3 cm) neuroendocrine liver metastases, radiofrequency ablation,
cryoablation, microwave ablation is most often used as an adjunct to surgical
resection. Another suitable option for initial treatment in these advanced
stages is liver-directed therapy, such as: hepatic arterial embolization,
chemoembolization or radioembolization. However, the survival benefit of this
treatment is less clear. In this case, it was performed, but then tried again
without success, when the disease progressed (32).
Hepatic arterial
embolization is frequently applied as a palliative technique in patients with a
metastatic neuroendocrine tumour of hepatic predominance who are not candidates
for surgical resection, which was the case in our case. It is based on the principle
that liver tumours obtain most of their blood supply from the hepatic artery,
while healthy hepatocytes obtain it from the portal vein32.
In this patient, the
expression of somatostatin receptors in the pancreas and in liver metastases
allowed the use of lutetium, achieving a favourable initial response with
reduction of hypoglycaemia and tumour stabilization. However, over time, the
disease progressed, suggesting possible resistance to treatment. Like trans
arterial embolization that was used, but in a second attempt it was not
achieved.
When the patient began
to present with recurrent hypoglycaemia despite the administration of
subcutaneous octreotide every 8 hours, the possibility of a paradoxical
aggravation of hypoglycaemia due to inhibition of the secretion of
counterregulatory hormones was raised. However, this hypothesis was discarded,
as hypoglycaemia persisted even after treatment was discontinued.
Conclusions
Insulinoma
is the most common functional pancreatic neuroendocrine tumour that causes
hyper insulinemic hypoglycaemia. Early diagnosis and intervention, particularly
in patients with a history of neurological or psychiatric problems,
postprandial symptoms or rapid improvement in symptoms after treatment, can
lead to significantly better outcomes18.
The therapeutic management of malignant insulinomas is complex and requires a
multidisciplinary approach. Surgery is the treatment of choice in respectables
cases; however, in advanced stages, as in this case, palliative strategies are
used with the main objective of controlling symptoms and improving the
patient's quality of life.
References
14. Vella
A, Nathan DM. Insulinoma. En UpToDate, Rubinow K (Ed), Wolters Kluwer 2025.
28. Ang Chan J, Kulke M, Clancy T et al. Metastatic
gastroenteropancreatic neuroendocrine tumors: Local options to control tumor
growth and symptoms of hormone hypersecretion. En UpToDate, Sonali M S (Ed),
Wolters Kluwer 2025.