Parathyroid Carcinoma Presenting with Severe Primary Hyperparathyroidism and Osteolytic Skeletal Lesions: A Case Report
Abstract
Parathyroid carcinoma is an exceptionally rare
malignancy typically presenting with severe primary hyperparathyroidism.
Diagnosis is often difficult and relies mainly on histopathological evaluation.
Surgery remains the cornerstone of treatment.
We report the case of a 52-year-old man with chronic
right hip pain and anxiety disorder, who presented with progressive general
deterioration. Clinical examination revealed a firm, painless, anterior
left-lateral basocervical mass measuring approximately 6 cm. Laboratory
investigations showed marked hypercalcemia (144 mg/L) and severe
hyperparathyroidism with a parathyroid hormone (PTH) level of 1,737 pg/mL (39
times normal). Imaging studies demonstrated a large heterogeneous mass adjacent
to the lower pole of the left thyroid lobe, associated with multiple osteolytic
skeletal lesions.
A giant parathyroid adenoma was suspected and surgical
excision was performed after preoperative correction of hypercalcemia. The mass
extended into the retrosternal space without invasion of adjacent structures.
Intraoperative histology suggested adenoma or hyperplasia. Postoperatively,
calcium and PTH levels normalized. However, definitive histopathological
analysis confirmed parathyroid carcinoma, showing capsular invasion and
vascular emboli.
At the six-month follow-up, the patient showed
significant improvement in bone pain, with no evidence of recurrence and normal
calcium levels. This case highlights the diagnostic difficulty of parathyroid
carcinoma and underscores the need for long-term surveillance due to the risks
of recurrence and metastasis.
Keywords: Parathyroid; Neoplasms; Carcinoma;
Hyperparathyroidism; Primary; Hypercalcemia; Parathyroidectomy
Introduction
Parathyroid
carcinoma (PC) is an exceptionally rare endocrine malignancy, accounting for
fewer than 1% of all cases of primary hyperparathyroidism (pHPT)1. The condition was first documented in
1904 by the Swiss surgeon de Quervain2
in a patient presenting with a non-functioning parathyroid lesion. Since this
initial description, the global literature has progressively expanded our
understanding of this uncommon neoplasm.
In
this article, we describe a case of parathyroid carcinoma in a patient with
persistent pHPT, reported in accordance with the SCARE criteria3 and provide a concise review of the
relevant literature.
Presentation of Case
A
52-year-old man with a history of right hip pain and anxiety disorder for the
past three years presented with progressive deterioration of his general
condition. Physical examination revealed a firm, painless, anterior
left-lateral Baso cervical mass, mobile on swallowing, measuring approximately
6 cm in its greatest dimension, with a non-palpable inferior border (Figure
1). No cervical lymphadenopathy was detected and vocal cord mobility was
preserved.
Figure
1: Physical examination revealed anterior left-lateral Baso cervical mass,
mobile on swallowing, measuring approximately 6 cm in its greatest dimension,
with a non-palpable inferior border
Laboratory
investigations demonstrated marked hypercalcemia (144 mg/L) and severe
hyperparathyroidism, with an extremely elevated parathyroid hormone (PTH) level
of 1,737 pg/Ml-approximately 39 times the upper limit of normal. Standard
skeletal radiographs revealed diffuse bone demineralization, predominantly
affecting the iliac bones, femoral necks and the right femoral diaphysis.
Cervical
ultrasonography identified a heterogeneous, hypoechoic and hypervascular mass
located adjacent to the lower pole of the left thyroid lobe, measuring 62 × 53
× 42 mm. Cervico-thoracic computed tomography (CT) confirmed a well-defined,
solid-cystic oval lesion beneath the left thyroid lobe, with smooth margins, measuring
54 × 45 mm and extending 63 mm in height. The mass showed close anatomical
relations with the left common carotid artery, situated anterior to it.
Additionally, multiple costal and vertebral osteolytic lesions were noted (Figure
2). Abdominopelvic CT revealed multiple osteolytic lesions involving the
spine and pelvis.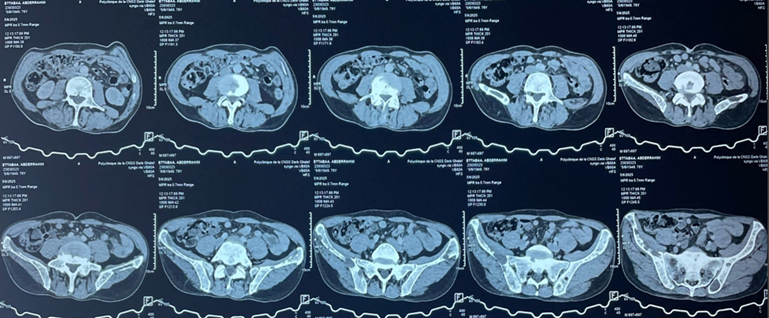
Figure
2: Axial abdominopelvic CT scan showing osteolytic bone lesions involving
the spine and the pelvis
Based
on these findings, a parathyroid adenoma was suspected and surgical excision
was planned. Preoperative management included intravenous rehydration with
isotonic saline and administration of bisphosphonates to correct the
hypercalcemia.
Intraoperatively,
the mass was found to be plunging into the retrosternal space, with no evidence
of infiltration or continuity with the lower pole of the left thyroid lobe,
which appeared macroscopically normal. A left inferior parathyroidectomy was
performed and the excised specimen was sent for intraoperative
histopathological examination, which suggested either an adenoma or parathyroid
hyperplasia without features of malignancy.
The
immediate postoperative course was uneventful. Biochemical assays on
postoperative day 1 demonstrated normalization of serum calcium (103 mmol/L)
and PTH levels (44 pg/mL).
Definitive
histopathological analysis established the diagnosis of parathyroid carcinoma,
characterized by an encapsulated malignant proliferation composed of
parathyroid cells arranged in diffuse and nodular patterns, separated by
fibrous septa. The tumor cells were monomorphic, with abundant cytoplasm and
mildly atypical round nuclei. Mitotic activity was moderate (three mitoses per
ten high-power fields). Areas of capsular invasion and vascular tumor emboli
were also identified.
At
the six-month follow-up, the patient showed significant improvement in bone
pain, with no clinical or ultrasonographic evidence of local recurrence.
Laboratory findings confirmed normalization of serum calcium levels.
Discussion
Parathyroid
carcinoma (PC) is an extremely rare endocrine malignancy, representing less
than 0.005% of all cancers and accounting for approximately 0.5-4% of primary
hyperparathyroidism cases, with significant geographical variation reaching up
to 5% in Japan4,5. In Western
countries, PC usually accounts for less than 1% of pHPT cases6. Its incidence is estimated at 4-6 cases
per 10 million inhabitants per year and a large American series reported 286
cases over 10 years7,8. Because
of its rarity and lack of specific clinical and biological signs, PC is
frequently misdiagnosed as benign primary hyperparathyroidism and is often
diagnosed only postoperatively4,7,9,1.
The
etiology of PC remains poorly understood, although several environmental and
genetic factors have been implicated10,7,9.
Neck irradiation, particularly at a young age, increases the risk of
parathyroid neoplasia5,6.
Chromosomal abnormalities-including 1p, 4q, 13q losses and 1q, 9q, 16p, Xq
gains-have been reported11 and
cyclin D1 overexpression is found in most tumors12.
Parathyroid carcinoma also shows a strong association with
hyperparathyroidism–jaw tumor syndrome13.
Additional genetic abnormalities, such as RB, p53, BRCA2 and PRAD1 mutations,
have been described13.
Most
PCs are functioning tumors causing severe hypercalcemia, presenting with
fatigue, weakness, weight loss, anorexia, psychiatric symptoms,
gastrointestinal complaints, nephrolithiasis and bone lesions including brown
tumors10,14,15. Renal and
skeletal involvement is common at presentation16.
Dysphonia and dysphagia, resulting from recurrent laryngeal nerve invasion, are
highly suggestive of malignancy10.
Marked hypercalcemia-often above 3.5 mmol/L-is frequently observed11,17. Non-functioning carcinomas are
extremely rare and typically present with advanced local disease7,15,18.
Imaging
plays a key role in the evaluation of PC. Cervical ultrasound may show
lobulated hypoechoic lesions with irregular margins, intra-lesional
calcifications or infiltration of adjacent tissues-features suggestive of
malignancy10,15. Negative
predictive features include a thick capsule, ovoid shape or absence of
intratumoral vascularity10.
Ultrasound sensitivity ranges from 50% to 90%15.
Tc-99m sestamibi scintigraphy is useful for localization but cannot distinguish
adenoma from carcinoma; however, it may detect lymph-node or distant metastases15,19. CT and MRI offer better visualization
of soft tissue invasion and nodal involvement14,20.
FDG-PET may show uptake in brown tumors, which can mimic metastasis21. Fine-needle aspiration cytology is not
recommended due to false negatives and risk of capsular rupture22,23.
Intraoperatively,
PC typically appears as a firm, lobulated mass with a dense gray-white fibrous
capsule adherent to surrounding tissues, making dissection difficult17,24. Tumors are usually large (>3 cm)
and may involve adjacent structures25.
Histopathological diagnosis is difficult. Classic criteria include trabecular
architecture, fibrous bands, mitotic activity and capsular or vascular invasion7,1,26, but these features are not specific
and may also occur in benign lesions27.
Surgery
is the mainstay treatment for PC. Recommended management includes en bloc
resection of the tumor with ipsilateral thyroid lobectomy and excision of
involved lymph nodes14,15,28.
Complete excision provides the best chance of cure, while incomplete resection
is associated with recurrence7,14,15,24.
Avoiding capsular rupture is essential to prevent tumor seeding10. Lateral lymph-node dissection is
recommended only when nodal metastases are present28,29.
Although PC is traditionally considered radioresistant, radiotherapy may
improve local control in selected cases17,20.
Chemotherapy has not shown proven benefit7,10.
Recurrence
is frequent, occurring in 25% to 60% of cases within the first 2-5 years7,30. Late recurrences, sometimes beyond 20
years, have been reported, requiring prolonged follow-up7. Recurrence often presents with rising
serum calcium and PTH levels and may involve local, regional or distant
metastases31. Follow-up includes
physical examination and serial monitoring of calcium and PTH13. Management of hypercalcemia may require
loop diuretics, dialysis or bisphosphonates10,14.
References
1. Shane E. Parathyroid carcinoma. J Clin
Endocrinol Metab 2001;86(2):485-493.
2. De
Quevain F. Malignant aberrant parathyroid. Dtsch Z Fuer Chir 1904;100:334-352.
5. Boudiaf
DE, Bouache MA, Kourtiche AS, Ouahioune W. Le carcinome parathyroïdien:
l’énigme diagnostique. Ann Endocrinol 2015;76(4):517-518.
14. Givi
B, Shah JP. Parathyroid Carcinoma. Clinical Oncology 2010;22:498-507.
15. Akoubkova
S, Vokurka J, Cap J, Ryska A. Parathyroid carcinoma: clinical presentation and
treatment. International Congress Series 2003;1240:991-995.
16. Koea
JB, Shaw JHF. Parathyroid cancer: biology and management. Surg Oncol 1999;8:155-165.
17. Trésallet
C, Royer B, Menegaux F. Cancer parathyroïdien. EMC Endocrinologie-Nutrition
2008.
26. Schantz
Z, Castleman B. Parathyroid carcinoma: a study of 70 cases. Cancer
1973;31(3):600-605.
28. Kassahun
WT, Jonas S. Focus on parathyroid carcinoma. Int J Surg 2011;9:13-19.
31. Kebebew E. Parathyroid carcinoma. Curr Treat
Options Oncol 2001;2:347-354.