Seven-Compartment Fasciotomy for Venous-Driven Acute Compartment Syndrome Secondary to Iliofemoral Deep Venous Thrombosis: A Case Report
Abstract
Acute compartment syndrome (ACS) of the lower limb is a
surgical emergency that can rapidly lead to irreversible neurovascular injury
and limb loss. This report describes a 40-year-old man with rapidly progressive
swelling, cyanosis, severe pain and evolving foot drop in the setting of
extensive iliofemoral deep venous thrombosis and prothrombotic comorbidities
(systemic lupus erythematosus, chronic myeloid leukemia, prior venous
thromboembolism and exogenous testosterone). Diagnosis was made clinically with
duplex confirmation of proximal venous outflow obstruction. The surgical team
performed an emergent seven-compartment fasciotomy, decompressing all three
thigh compartments and all four lower-leg compartments, verified
intraoperatively by visual and tactile findings. Wounds were managed open with
negative-pressure therapy and a planned second look; anticoagulation began once
hemostasis was secure and culture-directed antibiotics were given after
Citrobacter koseri was isolated. Limb salvage was achieved, with short-term
follow-up focused on wound progression, anticoagulation and neurologic
rehabilitation. This case highlights venous-driven, limb-wide ACS and provides
a practical approach when swelling spans the thigh and lower leg.
Keywords: Compartment syndromes; Fasciotomy; Venous
thrombosis; Lower extremity; Iliofemoral vein
Introduction
Acute compartment syndrome is a time-critical surgical
emergency in which elevated intercompartmental pressure impairs perfusion and
rapidly threatens limb viability1,2. The leg is the most frequently involved
site and population estimates suggest an incidence of about 7.3 per 100,000 in
men and 0.7 per 100,000 in women2. High-energy fractures, particularly of the
tibia, are common precipitants and timely fasciotomy is central to limb
salvage3,4. When the examination is equivocal, intercompartmental pressure
assessment can support decision-making, with a commonly cited differential
threshold near 30 mm Hg, but clinical judgment remains paramount1-3.
Beyond fracture and high-energy trauma, acute compartment
syndrome can follow vascular injury, ischemia-reperfusion, prolonged
compression and, more rarely, iliofemoral deep venous thrombosis with severe
venous hypertension, sometimes presenting as phlegmasia cerulea dolens5-7. In
such presentations, rapidly progressive swelling, pain out of proportion and
evolving neurologic deficit may precede overt ischemic skin changes; management
should prioritize timely decompression rather than extended imaging work-ups
that risk delay2,3.
We report a rare scenario requiring decompression of all
three thigh compartments and all four leg compartments for limb salvage.
Seven-compartment lower-limb releases have been described only in isolated case
reports, underscoring the importance of early recognition and comprehensive
fasciotomy when swelling spans both thigh and leg8. This report details the
clinical presentation, rationale for broad decompression, perioperative
decisions including anticoagulation and wound strategy and short-term outcomes.
Case Presentation
A 40-year-old man was referred from a private clinic for
rapidly progressive right lower-extremity swelling and pain after a soft-tissue
injury to the thigh. Over several days he developed marked edema, increasing
tension in the thigh and leg and worsening pain despite analgesia. On arrival
he was visibly cyanotic in the affected limb. Neurologic examination
demonstrated a complete foot drop with 0/5 ankle dorsiflexion strength and
inability to actively flex the ankle. Distal pulses were palpable and capillary
refill was delayed (Figure 1).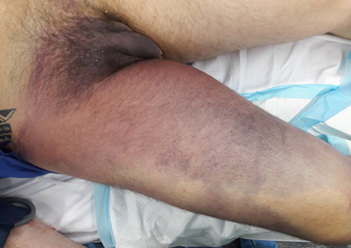
Figure 1. Preoperative discoloration
of the right thigh and groin.
Marked purple-red discoloration extends from the distal
thigh through the groin to the right hip, consistent with extensive venous
congestion due to iliofemoral thrombosis and evolving compartment syndrome
involving the proximal compartments.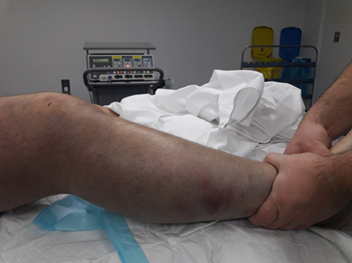
Figure 2. Clinical appearance of the
right lower limb prior to fasciotomy.
The surgeon demonstrates pallor and diffuse purple
discoloration of the tense, edematous leg. The limb was firm to palpation with
absent distal pulses, consistent with acute compartment syndrome and severe
venous hypertension.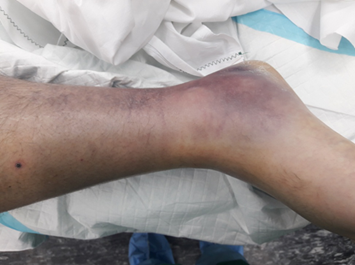
Figure 3. Right ankle showing severe
dependent edema and ecchymosis.
Marked swelling and purple discoloration are evident around
the ankle and distal leg, consistent with dependent venous congestion and
ischemic changes prior to decompression.
Bedside duplex ultrasonography of the right lower extremity showed an occlusive femoral deep venous thrombosis with suspected proximal extension into the iliac system. Given the clinical picture of escalating pain, tense compartments spanning the thigh and leg and evolving neurologic deficit, acute compartment syndrome was diagnosed and the patient was taken emergently to the operating room (Figure 4).
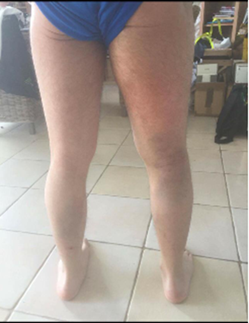
Figure 4. Posterior view of
bilateral lower limbs.
Diffuse erythema and swelling of the right buttock, thigh,
and lower leg are visible compared to the contralateral limb, reflecting
extensive soft-tissue involvement secondary to iliofemoral outflow obstruction.
Fasciotomy was performed to
decompress all seven compartments of the involved limb. The three thigh
compartments were released using a lateral approach with extension as needed to
ensure full decompression. The four compartments of the leg were released using
a standard two-incision technique, ensuring thorough decompression of the deep
posterior compartment. Intraoperatively, there was tense fascia with marked
intramuscular swelling and hematoma; evacuation of large clot burdens was
required. Multiple specimens were sent for culture and sensitivity (Figure 5). Wounds were left open and
two drains were placed. Negative-pressure wound therapy and staged closure were
planned.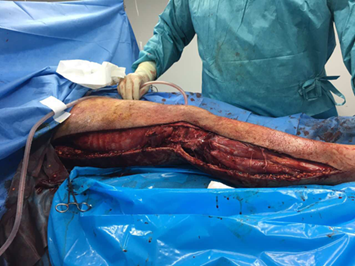
Figure 5. Intraoperative view of the
right-sided fasciotomy.
Comprehensive decompression was achieved through a
continuous lateral incision extending from the greater trochanter to the
external malleolus, allowing release of all seven compartments. The photograph
demonstrates exposure of underlying fascia and musculature under high tension
along the full length of the limb.
Postoperatively, targeted wound
management was provided. Tissue culture later grew Citrobacter koserii and
antibiotic therapy was tailored to the organism’s susceptibilities based on the
antibiogram. Vascular surgery was consulted for thrombosis management, with
systemic anticoagulation initiated once haemostasis was secured. The remainder
of the postoperative course was uncomplicated.
Past medical history included
systemic lupus erythematosus, chronic myeloid leukaemia, a prior venous
thromboembolism and a previous methicillin-resistant Staphylococcus aureus
infection of the upper extremity. The patient also reported exogenous
testosterone use.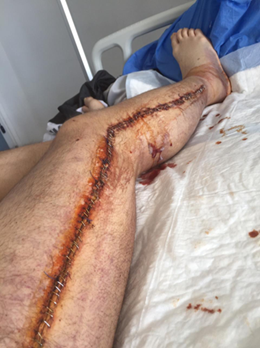
Figure 6: Post-fasciotomy closure of
the right lower limb.
Stapled skin closure following staged wound management
extends from the greater trochanter to the external malleolus along the lateral
aspect of the limb. The wound surface was treated with povidone-iodine to
reduce microbial contamination before dressing application.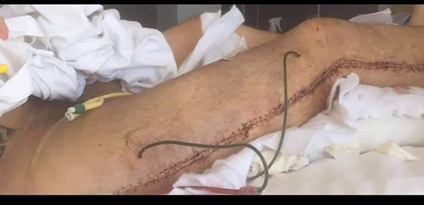
Figure 7: Placement of postoperative
drains in the right lower leg.
Two closed-suction drains were positioned anteriorly in the
proximal thigh following decompression extending from the greater trochanter to
the external malleolus. Drain placement was performed to prevent postoperative
fluid accumulation, reduce soft-tissue tension in the proximal compartments and
facilitate wound healing during the early postoperative period.
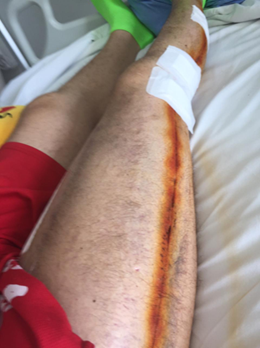
Figure 8: Follow-up appearance after
staple removal.
Postoperative healing is evident along the previous
fasciotomy line extending from the thigh to the ankle. Re-epithelialization and
soft-tissue recovery are visible, with decreased swelling and restored contour
of the right lower limb.
His past medical history includes
systemic lupus erythematosus with concomitant arthritis, myositis, neutropenia,
nephritis, cardiomyopathy, Raynaud's syndrome, alopecia, mouth ulcers, chronic
myeloid leukaemia, deep vein thrombosis of the upper extremity, MRSA. He also
used testosterone injections.
Key laboratory findings on
presentation were notable for marked leucocytosis with neutrophilia, severe
hyponatremia, mild hyperkalaemia, elevated transaminases, markedly increased
lactate dehydrogenase, elevated CK-MB, very high C-reactive protein and an
extreme creatine phosphokinase elevation consistent with rhabdomyolysis.
Representative laboratory values included:
●
WBC: 24,220
●
Neutrophils: 19,180
●
Lymphocytes: 2,330
●
Monocytes: 2.690
●
Hemoglobin: 7.4
●
Urea: 91
●
Creatinine: 2.87
●
Sodium: 121
●
Potassum: 5.20
●
SGPT: 123
●
SGOT: 301
●
Lactate Dehydrenase: 1,579
●
CK-MB: 105.2
●
CRP: 96.24
●
CPK 12,613
Anatomy and operative considerations
The lower limb contains three thigh
compartments (anterior, medial, posterior) and four lower leg compartments
(anterior, lateral, superficial posterior, deep posterior). In diffuse swelling
from iliofemoral venous thrombosis, pressure can rise across contiguous
segments, so selective releases risk leaving ischemic muscle behind. The
surgeons therefore planned comprehensive decompression from thigh to foot, with
special attention to the deep posterior compartment of the leg and the medial
thigh, which are commonly under-released in extensive edema.
Thigh
The surgeons used a lateral incision
to decompress the anterior and posterior compartments and extended the exposure
proximally or distally as needed to ensure full release. The medial compartment
was assessed independently to avoid missing adductor compartment hypertension
while protecting the femoral vessels and nerve. When fascia over the adductors
remained tight, a separate medial release was performed.
Lower leg
A standard two-incision approach was
used for the lower leg. Through the anterolateral incision, the surgeons
released the anterior and lateral compartments while identifying and protecting
the superficial peroneal nerve. Through a posteromedial incision, they released
the superficial posterior compartment and then opened the deep posterior
compartment along the posteromedial tibia. The soleus bridge was divided, the
fascia over tibialis posterior was opened along its length and free excursion
of flexor hallucis longus was confirmed.
Verification and postoperative strategy
At each level, the team confirmed
complete decompression by direct visualization of muscle herniation, palpation
of softened compartments and reassessment of tibial and peroneal nerve
function. Wounds were left open with negative-pressure therapy, with a planned
return to the operating room within 24 to 72 hours for reassessment and
debridement as needed. Staged closure was achieved using dermatotraction or
delayed primary closure when tissues permitted and anticoagulation decisions
were coordinated with soft-tissue status and haemostasis.
Discussion
How the patient’s comorbidities likely converged to cause
seven-compartment ACS
This presentation can be explained
by several prothrombotic influences acting together to produce a large
iliofemoral deep venous thrombosis, marked venous hypertension and rapid limb
swelling from thigh to foot. Systemic lupus erythematosus, chronic myeloid
leukemia with possible treatment effects, a prior venous thromboembolism and
exogenous testosterone likely increased clot formation and promoted proximal
propagation once thrombosis began. High venous pressures accelerate
interstitial fluid accumulation, lymphatic clearance is overwhelmed and
compartment pressures rise in closed fascial spaces. As perfusion pressure
falls, muscle and nerve become ischemic in parallel compartments, which fits
the need for comprehensive seven-compartment decompression in this case6,9,10.
Systemic lupus erythematosus and antiphospholipid pathways
People with SLE have higher venous
thromboembolism rates, especially when antiphospholipid antibodies are present.
Mechanistically, antiphospholipid antibodies activate endothelium and platelets
through beta-2 glycoprotein I complexes, promote neutrophil extracellular traps
and amplify complement, which increases tissue factor expression and thrombin
generation. These changes favor rapid formation and extension of fibrin-rich
thrombi in the iliac and femoral veins, raising venous pressure throughout the
limb. In an SLE patient with a large iliofemoral DVT, early testing for lupus
anticoagulant, anticardiolipin and anti-beta-2 glycoprotein I antibodies is
useful because confirmed antiphospholipid syndrome can alter intensity and
duration of anticoagulation and supports closer surveillance for recurrence11-17.
Chronic myeloid leukaemia and therapy-related risk
Cancer increases thrombotic risk
through inflammation, procoagulant microparticles and altered blood counts. In
CML, baseline venous risk is lower than in other myeloproliferative neoplasms,
but treatment can modify that risk. Tyrosine kinase inhibitors have different
vascular profiles. Ponatinib and to a lesser extent nilotinib and dasatinib,
are linked to higher rates of arterial events and some venous thrombosis
compared with imatinib. Endothelial stress, metabolic effects and platelet
reactivity may all tilt the balance toward thrombosis and facilitate proximal
clot propagation. If this patient was receiving a higher-risk TKI, that
exposure could have compounded the SLE or antiphospholipid contribution and
intensified venous outflow obstruction18-21.
Exogenous testosterone as an amplifier
Observational reports connect
testosterone therapy with venous thromboembolism, although meta-analyses show
mixed results and the cardiovascular outcomes data are reassuring when therapy
is appropriately indicated. Testosterone can raise haematocrit and viscosity
and may influence platelet function and coagulation proteins. In a patient who
already has strong prothrombotic drivers such as SLE or active malignancy,
these changes can lower the threshold for thrombosis and support a larger
thrombus burden once clotting begins. During the acute event, it is reasonable
to reassess the necessity and timing of therapy while anticoagulation and wound
management are being coordinated22-25.
Effect of prior venous thromboembolism and proximal clot
location
A previous DVT raises the risk of
recurrence, particularly after proximal events. Residual venous obstruction and
valve damage reduce venous reserve and make any new thrombus more likely to
generate large pressure gradients. When a recurrent event involves the iliac
and femoral segments, venous hypertension extends through both thigh and leg,
accelerating edema and broadening the risk of compartment syndrome across
multiple anatomical compartments26.
How these risks combine to trigger compartment syndrome
The unifying pathway begins with
iliofemoral outflow obstruction that drives venous hydrostatic pressure upward.
Interstitial edema accelerates, intercompartmental pressure increases and
capillary flow falls. Perfusion pressure can be understood simply as arterial
pressure minus intercompartmental pressure. When those gradient narrows, tissue
oxygen delivery drops and ischemia advances. Severe cases also develop
secondary impairment of arterial inflow, a pattern similar to phlegmasia
cerulea dolens. This sequence explains simultaneous compromise of thigh and leg
compartments and supports early, comprehensive decompression for limb salvage6,9,10.
Conclusion
This case describes acute
compartment syndrome involving all three thigh compartments and all four
compartments of the lower leg in the setting of extensive iliofemoral deep
venous thrombosis. The presentation is best understood as the product of
several prothrombotic influences acting together, including systemic lupus
erythematosus, chronic myeloid leukaemia with possible treatment effects, a
prior venous thromboembolism and exogenous testosterone. The resulting venous
hypertension produced rapid, limb-wide edema and a critical fall in perfusion
pressure across multiple compartments at once.
Management focused on timely
recognition and decisive, comprehensive decompression. The surgical team
planned release of all seven compartments from the outset, with special
attention to the deep posterior compartment of the lower leg and the medial
thigh, verified complete decompression intraoperatively and used staged wound
care with a planned second look. Anticoagulation was coordinated with
soft-tissue status and culture results guided antibiotic selection. This
approach reflects a practical strategy when swelling spans the thigh and lower
leg and neurologic deficits are evolving.
The clinical implications are
straightforward. First, when a patient with strong thrombotic risk presents
with large-territory iliofemoral thrombosis and tense swelling, clinicians
should reassess compartments frequently and prioritize decompression over
extended diagnostic workups when the examination is convincing. Second, when
the anatomic extent of swelling is broad, proactive seven-compartment planning
reduces the risk of under-release. Third, evaluation for antiphospholipid
antibodies in systemic lupus erythematosus, documentation of tyrosine kinase
inhibitor exposure in chronic myeloid leukaemia and reconsideration of
exogenous testosterone can refine long-term anticoagulation and recurrence
prevention. Finally, early rehabilitation and close follow-up are essential
given the risk of persistent neurologic deficits.
Taken together, the case emphasizes
that venous-driven compartment syndrome can be as time-critical as
fracture-related disease. Recognizing the comorbidity stack, committing to
comprehensive decompression when indicated and aligning anticoagulation with
wound strategy are the key steps that support limb salvage and functional
recovery.
Declarations
Ethics approval was not required for
this case report. The patient provided informed consent to participate and
anonymized medical data were collected and analysed for educational and
publication purposes. All data supporting the findings of this case are
contained within the manuscript and no additional datasets were generated. The
author declares no conflicts of interest and received no external funding for
this work. All contributions, including data analysis, literature review and
manuscript preparation, were undertaken by the author.
Written consent for publication of
anonymized clinical details and images was obtained from the patient through
the Orthopaedic Surgery Department of Paphos General Hospital. The case was
first presented at an orthopaedic conference and afterward the anonymized
clinical information and relevant explanations were provided to the author for
preparation of this report. This information was already in the public domain
prior to submission of the manuscript for publication.
Acknowledgments
I extend my sincere thanks to the
Orthopaedic Surgery Department of Paphos General Hospital for their generous
support in developing this case report. Their detailed insights into the
clinical background, surgical rationale and procedural approach greatly
deepened my understanding of complex compartment syndrome management. I am
especially grateful for their provision of operative photographs, laboratory
data and imaging studies, which enriched the clinical narrative and educational
value of this manuscript. Their commitment to transparency and teaching
exemplifies the collaborative spirit that advances clinical practice.
References
2. Cone J, Inaba K. Lower extremity compartment
syndrome. Trauma Surgery Acute Care Open 2017;2(1):000094.