The Unwelcome Guest: ST-Elevation Myocardial Infarction After a Femoral Surgery Invitation
Abstract
Background: Perioperative myocardial infarction (PMI) is a significant cause of morbidity and mortality in patients undergoing non-cardiac surgery, particularly among elderly individuals with cardiovascular risk factors. Despite advances in medical care, PMI remains a critical challenge due to its complex pathophysiology and atypical presentation in the postoperative setting.
Case
presentation: We report the case of a 64-year-old woman
with type 2 diabetes mellitus admitted for total hip arthroplasty (THA)
following a left femoral neck fracture. On postoperative day 2, she developed
acute chest pain and complete atrioventricular block (AVB). Electrocardiography
revealed ST-segment elevation in the inferior leads consistent with an acute
inferior ST-elevation myocardial infarction. Given the complete AVB, a
temporary transvenous pacing wire was promptly placed. Emergent coronary angiography
demonstrated a thrombotic occlusion of the second segment of the right coronary
artery (RCA), which was successfully treated with percutaneous coronary
intervention (PCI) and placement of a drug-eluting stent (DES). Post-procedure,
the patient’s AVB resolved, allowing the removal of the temporary pacing system
and was managed with dual antiplatelet therapy, statins and beta-blockers. She
was discharged on day 7 without further complications.
Discussion: PMI arises from a multifactorial interplay of perioperative stressors, including sympathetic activation, inflammation and a hypercoagulable state, leading to plaque destabilization and acute coronary events. This case underscores the importance of vigilant monitoring, early diagnosis using biomarkers and ECG and timely intervention, particularly in high-risk surgical patients. The use of PCI in the perioperative setting poses unique challenges, including balancing antiplatelet therapy with bleeding risks. Multidisciplinary management and preoperative risk stratification are critical for improving outcomes.
Conclusion:
PMI remains a significant complication in non-cardiac surgery, requiring prompt
recognition and coordinated care to mitigate its impact on morbidity, mortality
and healthcare costs. This case highlights the importance of personalized,
multidisciplinary approaches to optimize perioperative outcomes in high-risk
patients.
Keywords: Perioperative myocardial infarction; Cardiovascular risk factors; Non-cardiac surgery
The perioperative state induces a cascade of physiological changes, including systemic inflammation, hypercoagulability and sympathetic activation. These factors, combined with underlying coronary artery disease (CAD), create a perfect storm for plaque destabilization, thrombus formation and myocardial ischemia.
Patients with diabetes are particularly vulnerable to such events due to chronic endothelial dysfunction, accelerated atherosclerosis and a heightened pro-thrombotic state. Orthopedic procedures, such as total hip arthroplasty (THA), carry an increased risk due to the systemic stress induced by surgical trauma, blood loss and prolonged immobility. This case report describes the occurrence of STEMI on postoperative day two (POD 2) in a diabetic patient after hip fracture surgery. It explores the underlying mechanisms, diagnostic complexities and therapeutic approach, with a focus on the unique challenges presented in such cases.
Case
Presentation
A
64-year-old woman with a history of type 2 diabetes mellitus was admitted for
total hip arthroplasty (THA) following a left femoral neck fracture (Figure1). The patient had no known
history of cardiovascular disease and reported no angina or dyspnea.
Preoperative investigations, including routine blood work, chest radiography
and an electrocardiogram (ECG), were unremarkable.
The surgery was performed under regional anesthesia and proceeded uneventfully. The patient was stable during the immediate postoperative period. However, on postoperative day (POD) 2, she developed sudden-onset, severe retrosternal chest pain radiating to the left arm. The pain was associated with diaphoresis and a feeling of impending doom.
Clinical examination revealed a regular rhythm (heart rate 75 bpm), with a blood pressure of 129/60 mmHg. An ECG showed ST-segment elevation in inferior leads (II, III, aVF), specifically in the inferobasal territory, with reciprocal ST-segment depression in anterior leads (V1-V3) and lateral leads (I, aVL), along with a complete atrioventricular block (AVB) These findings were consistent with a postero-inferior STEMI extended to the right ventricle (Figure 2). Blood tests revealed elevated troponin levels (peak 15 ng/mL; normal <0.01 ng/mL), indicating acute myocardial injury. An echocardiogram demonstrated preserved left ventricular ejection fraction (LVEF) at 55-60%, with hypokinesia localized to the inferior wall.
Given the complete AVB, a temporary transvenous pacing wire was promptly placed to ensure adequate cardiac output.
The patient was transferred to the catheterization laboratory for emergent coronary angiography. This revealed a complete thrombotic occlusion of the first segment of the right coronary artery (RCA) (Figure 3). A percutaneous coronary intervention (PCI) was performed, with successful placement of a drug-eluting stent (DES), achieving TIMI grade 3 flow. (Figure 4).
Following
the PCI, the patient's chest pain resolved and the AVB spontaneously reverted
to normal sinus rhythm within hours, allowing for the removal of the temporary
pacing system. However, during the post-operative evaluation, the patient was
found to have developed postoperative anemia with a hemoglobin level of 8 g/dL,
likely due to blood loss during surgery and the inflammatory response. This
necessitated the transfusion of 2 units of red blood cells to restore her
hemoglobin level to 10 g/dL. She was started on dual antiplatelet therapy
(aspirin and clopidogrel), high-intensity statins, beta-blockers and optimized
glycemic control. The patient was discharged on day seven with no further
complications.
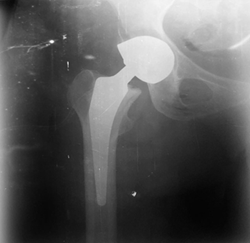
Figure 1: Total hip arthroplasty (THA)
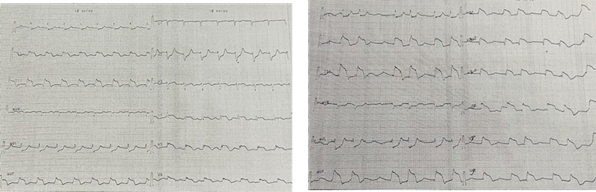
Figure 2: Postero-inferior STEMI extended to the right
ventricle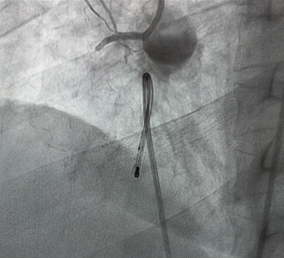
Figure 3: Coronary angiogram showing an occlusion of CDI
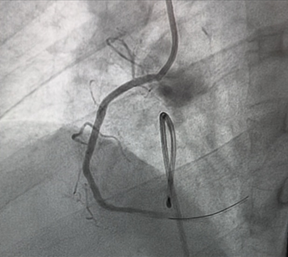
Figure 4: Percutaneous coronary intervention of CDI with
successful result
Discussion
The incidence of elderly patients with
cardiovascular disease undergoing non-cardiac surgery continues to rise,
accompanied by an increasing prevalence of perioperative cardiovascular
complications. These complications, particularly perioperative myocardial
infarction (PMI), are a significant public health concern, as they not only
lead to extended hospital stays and increased healthcare costs but also
contribute to high morbidity and mortality rates. Despite considerable
advancements in healthcare, myocardial ischemia remains the most common
perioperative complication in non-cardiac surgeries, particularly in high-risk
populations1.
PMI is a severe and potentially fatal complication driven by a complex interplay of pathophysiological mechanisms. It often results from an imbalance between myocardial oxygen supply and demand, exacerbated by perioperative stress. Surgical trauma activates the sympathetic nervous system, leading to tachycardia and hypertension, which markedly increase myocardial oxygen demand. Simultaneously, factors such as intraoperative or postoperative hypotension, hypovolemia, hypoxemia or anemia, as observed in this case, can reduce oxygen supply, creating a critical mismatch that predisposes the myocardium to ischemia2,3.
Additionally, perioperative inflammation and the hypercoagulable state induced by surgical stress play a key role in destabilizing atherosclerotic plaques. This destabilization makes plaques prone to rupture or erosion, triggering thrombus formation and, in some instances, complete coronary artery occlusion, as observed in our patient. The resulting thrombotic event led to the acute inferior STEMI, confirmed by ST-segment elevation in inferior leads with reciprocal changes in the anterior and lateral leads. The associated complete atrioventricular block (AVB) is a hallmark of ischemia in the right coronary artery territory, reflecting its involvement in supplying the atrioventricular nodal tissue4.
In this case, our patient underwent orthopedic surgery which, according to ESC recommendations, was considered to be surgery with an intermediate risk of cardiovascular death and myocardial infarction at 30 days. For this reason, selected patients should be assessed by an integrated multidisciplinary specialist team including anaesthetists, cardiologists and surgeons and, where appropriate, an extended team (e.g. internists, intensivists, pulmonologists or geriatricians)5.
Clinical presentation of PMI is often atypical, particularly in the postoperative setting. Analgesics, sedation and the effects of advanced age and diabetes mellitus can mask classic symptoms such as chest pain. In fact, studies, such as those by Devereaux et al., show that the majority of PMIs (74.1%) occur within the first 48 hours postoperatively, with 65.3% being asymptomatic. This emphasizes the importance of vigilant monitoring through routine ECG changes and cardiac biomarkers to detect early signs of ischemia. In this case, the combination of identified symptoms and inferior STEMI findings allowed for timely diagnosis and intervention6.
Emergent percutaneous coronary intervention (PCI) is the gold standard for managing STEMI, even in the perioperative setting. In this patient, PCI effectively restored coronary flow, resolved the AVB and minimized myocardial damage. However, the perioperative setting presents unique challenges, particularly in balancing the need for dual antiplatelet therapy (DAPT) to prevent stent thrombosis with the increased risk of perioperative bleeding. Careful coordination between cardiologists and surgeons is essential to navigate these risks7.
Improving outcomes for PMI requires a proactive, multidisciplinary approach. Preoperative risk stratification using tools like the Revised Cardiac Risk Index (RCRI) is critical to identifying high-risk patients. Optimization of medical therapy with statins, beta-blockers and improved glycemic control is essential for stabilizing atherosclerotic plaques and reducing perioperative stress8. Furthermore, continuous postoperative monitoring with ECG and serial cardiac biomarker assessments enables early detection and intervention for myocardial ischemia. This case highlights the importance of integrating evidence-based management strategies and collaboration among specialties to address the complex interplay of factors contributing to PMI, ultimately improving both short- and long-term patient outcomes9.
Conclusion
In
conclusion, perioperative myocardial infarction (PMI) is a critical
complication that significantly impacts the prognosis of patients undergoing
noncardiac surgery, particularly those with predisposing risk factors such as
diabetes and atherosclerosis. The pathophysiology of PMI involves a complex
interplay of increased myocardial demand, reduced oxygen supply and systemic
inflammation, which can destabilize atherosclerotic plaques and trigger acute
coronary events. Timely recognition and intervention, such as percutaneous
coronary intervention (PCI), are essential to restoring coronary perfusion and
improving short-term survival. This case underscores the importance of
preoperative risk stratification, vigilant monitoring and a multidisciplinary
approach to perioperative care in reducing the morbidity and mortality
associated with PMI. Early detection and prompt management can substantially
improve outcomes, highlighting the need for proactive cardiac management in
high-risk surgical patients.
Conflict
of interest
None.
References
1. Mackey WC, Fleisher LA,
Haider S, et al. Perioperative myocardial ischemic injury in high-risk vascular
surgery patients: Incidence and clinical significance in a prospective clinical
trial. J Vasc Surg 2006;43:533-538.
2. Devereaux PJ, Xavier D,
Pogue J, et al. Characteristics and short-term prognosis of perioperative
myocardial infarction in patients undergoing noncardiac surgery: a cohort
study. Ann Intern Med 2011;154(8):523-528.
3. Li SL, Wang DX, Wu XM, Li N, Xie YQ. Perioperative
acute myocardial infarction increases mortality following noncardiac
surgery. J Cardiothorac Vasc Anesth 2013;27(6):1277-1281.
4. Le Manach Y, Perel A,
Coriat P, Godet G, Bertrand M, Riou B. Early and delayed myocardial infarction
after abdominal aortic surgery. Anesthesiology 2005;102(5):885-891.
5. Kristensen SD, Knuuti J,
Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery:
cardiovascular assessment and management: The Joint Task Force on non cardiac surgery: cardiovascular assessment and management of the European Society of
Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur
Heart J 2014;35(35):2383-2431.
6. Badner NH, Knill RL,
Brown JE, Novick TV, Gelb AW. Myocardial infarction after noncardiac surgery
published correction appears in Anesthesiology 1999;90(2):644.
7. Devereaux
PJ, Goldman L, Yusuf S, Gilbert K, Leslie K, Guyatt GH. Surveillance and
prevention of major perioperative ischemic cardiac events in patients
undergoing noncardiac surgery: a review. CMAJ 2005;173(7):779-788.
8. Don
Poldermans, Olaf Schouten, Jeroen Bax, Tamara A. Winkel, Reducing cardiac risk
in non-cardiac surgery: evidence from the DECREASE studies, European Heart
J
Supplements 2009:11:9-14
9. Ranjeva SL, Tung A,
Nagele P, Rubin DS. Morbidity and Mortality After Acute Myocardial Infarction
After Elective Major Noncardiac Surgery. J Cardiothorac Vasc Anesth2021;35(3):834-842.