Unilateral Tongue Angioedema Caused Bay Lopril: A Case Report
Abstract
Introduction
ACE
inhibitors are widely prescribed for anti-proteinuria, nephroprotection, cardio
protection or treatment of hypertension. Angioedema, one of the adverse effects
of ACE inhibitors, is not uncommon. It can sometimes be life-threatening.
Unilateral angioedema of the tongue due to ACE inhibitors is rarely described.
Case presentation
This is a 57-year-old patient (S. A.) with a history of chronic renal failure on hemodialysis for two years and hypertensive, initially treated with an ARB II for two years then replaced by an ACE inhibitor, lopril. Six months after the introduction of lopril, the patient presented with slight edema of the left half of the tongue with moderate glossitis 2 days after a CT scan injected with an iodinated contrast agent. The symptoms were linked to a probable allergy to iodinated PDC and the patient was treated with an antihistamine and intravenous corticosteroids with rapid regression in two days. Six months later, the patient presented with significant edema of the right half of the tongue with difficulty speaking and swallowing saliva with a state of anxiety and agitation, a hypertensive peak at 190/90 mm Hg and a correct arterial oxygen saturation in room air at 98%.
He was taken into emergency care with injection of hydrocortisone hemi succinate and nebulizations with adrenaline with the prescription of an antihistamine. The complete blood count did not show hypereosinophilia and specific IgE was negative for food products. The evolution was marked by a progressive regression of symptoms until disappearance in three days. The diagnosis was unilateral angioedema of the tongue due to ACE inhibitors and the causal treatment was permanently discontinued.
Discussion
ACE inhibitors are usually well tolerated by the majority of patients. However, 0.7% of patients taking ACE inhibitors develop angioedema. The time to onset of angioedema due to ACE inhibitors ranges from a few hours to several years after initiating treatment. Angioedema can also occur after treatment discontinuation. Bradykinin is considered the main factor contributing to the development of angioedema. The combination of an ACE inhibitor with certain drugs that increase bradykinin concentrations increases the risk of developing bradykinin-induced angioedema. Unilateral tongue involvement is rare and only a few case reports have been published documenting this condition. The reason for unilateral tongue edema is not well understood. The basic treatment of ACE inhibitor-induced angioedema consists of securing the upper airway and preparing for artificial ventilation if necessary. Corticosteroids and antihistamines are widely used in the management of these cases.
Conclusion
Unilateral angioedema of the tongue, which is less frequently described in the literature, among other things, should consider the use of ACE inhibitors.
Keywords: ACE inhibitor; Lopril; Unilateral angioedema of the tongue
Introduction
Angioedema in general may result from a mast cell-mediated reaction or be associated with an increase in bradykinin concentration, linked to a defect in its catabolism1.
It is clinically difficult to differentiate between the two mechanisms, particularly in the presence of a poor presentation or one made exclusively of angioedema, especially since there is currently no reliable biomarker to differentiate the two diagnoses. Angioedema most often presents as non-pruritic, sudden-onset hypodermal edema, located mainly on the face, tongue or upper airways2.
It can also be localized to the digestive mucosa and present in the form of abdominal pain of varying intensity. Angioedema can be of hereditary origin (associated or not with a deficiency in C1 inhibitor) or of drug origin by inhibition of enzymes which degrade bradykinin, in particular: the converting enzyme for angiotensin converting enzyme inhibitors (ACE) or angiotensin II receptor antagonists (ARB II) or dipeptidyl peptidase for gliptins. Other causes of angioedema, such as pollen-food allergy syndrome which is a cross-reaction mediated by immunoglobulin E type I to an antigen of plant origin, as it can be caused by an infection3,4.
ACE inhibitors are widely prescribed for anti-proteinuria, nephroprotection, cardio protection or treatment of hypertension. Angioedema, one of the adverse effects of ACE inhibitors, is not uncommon. It can sometimes be life-threatening. Angioedema due to ACE inhibitors is often localized to the ENT area. Unilateral angioedema of the tongue due to ACE inhibitors is rarely described.
We report in this study a case of a hemodialysis patient treated with an ARB II for 2 years and then with the ACE inhibitor lopril for 2 years. He presented with a severe attack of unilateral angioedema of the tongue with difficulty speaking and swallowing saliva, due to the adverse effect of lopril, requiring permanent discontinuation of the ACE inhibitors.
Case Presentation
This is a 57-year-old patient (S. A.) with a history of chronic renal failure due to familial nephropathy on hemodialysis for two years and hypertensive, initially treated with an ARB II for two years then replaced by an ACE inhibitor, lopril. Six months after the introduction of lopril, the patient presented with slight edema of the left half of the tongue with moderate glossitis 2 days after a CT scan injected with an iodinated contrast agent. The symptoms were linked to a probable allergy to iodinated PDC and the patient was treated with an antihistamine and intravenous corticosteroids with rapid regression in two days (Figure 1).
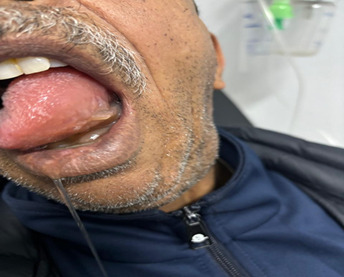
Figure 1: Pateint with antihistamine and intravenous corticosteroids with rapid
regression
Six months later, the patient presented with
significant edema of the right half of the tongue with difficulty speaking and
swallowing saliva with a state of anxiety and agitation, a hypertensive peak at
190/90 mm Hg and a correct arterial oxygen saturation in room air at 98%.
He was taken into emergency care with injection of
hydrocortisone hemi succinate and nebulization’s with adrenaline with the
prescription of an antihistamine. The complete blood count did not show hyper
eosinophilia and specific IgE was negative for food products. The evolution was
marked by a progressive regression of symptoms until disappearance in three
days. The diagnosis was unilateral angioedema of the tongue due to ACE
inhibitors and the causal treatment was permanently discontinued.
Discussion
ACE inhibitors are usually well tolerated by the
majority of patients. However, 0.7% of patients taking ACE inhibitors develop
angioedema5.
Other studies suggest that the incidence of angioedema
in patients treated with ACE inhibitors is estimated at 0.20%6. Estimates of cross-reactions between ACE
inhibitors and ARB II in different studies range from 7.7% to 50%3.
The time to onset of angioedema due to ACE inhibitors
ranges from a few hours to several years after initiating treatment, but the
majority of cases occur within the first three months following treatment
initiation7.
Cases of angioedema have been reported after 23 years
of continuous treatment6. In our
patient, angioedema appeared after two years of ACE inhibitor treatment and the
second attack was more severe than the first. Angioedema can also occur after
treatment discontinuation. In another published case, angioedema developed
after 32 years of continuous ACE inhibitor treatment and its recurrence was
observed 2 years after discontinuation8.
The attacks can become more and more frequent and/or
more and more severe as in our case, always with a predilection for the ENT
sector.
The combination of an ACE inhibitor or sartan with
certain drugs that increase bradykinin concentrations increases the risk of
developing bradykinin-induced angioedema. We noted in the literature the
publication of a case of unilateral periorbital angioedema induced by contrast
media, as well as another case of intestinal angioedema also induced by
contrast media9,10.
Another case of unilateral angioedema of the tongue
induced by paracetamol (Acetaminophen) has been published11.
Aspirin has also been described as an inducer of
angioedema of half the tongue in another publication12.
We wonder whether the injection of contrast agent in
our patient's case plays an additional role in the onset of ACE
inhibitor-induced angioedema during the first attack.
ACE inhibitor-induced angioedema is due to the effects
of ACE inhibitors on the renin-angiotensin-aldosterone system, which results in
increased angiotensin I and bradykinin levels due to a failure of bradykinin
degradation by inhibition of enzymes that degrade bradykinin by ACE inhibitors,
particularly the angiotensin-converting enzyme1.
Bradykinin is considered the main factor contributing
to the development of angioedema, as it causes vasodilation and swelling1. Unlike histamine-releasing angioedema,
bradykinin angioedema spontaneously regresses despite continued use of the
causative drug, often leading to the drug hypothesis not being considered.
However, the symptoms improved with the administration antihistamines;
therefore, an associated histamine mechanism could not be excluded1.
Unlike hereditary or acquired forms of
bradykinin-induced angioedema, C1 inhibitor (weight or functional) and C4
levels are normal.
The diagnosis of ACE inhibitor-induced angioedema is
generally based on clinical symptoms. Typical symptoms are localized angioedema
of the face, erythema without pruritus persisting for 24 to 72 hours, followed
by spontaneous remission2.
Unilateral tongue involvement is rare and only a few
case reports have been published documenting this condition. Our case
represents the second case of unilateral ACE inhibitor-induced angioedema
published in Africa after that of Julian Robert Paul Eloff despite the
frequency of hypertension and frequent use of ACE inhibitors suggesting the
rarity of the involvement or underestimation due to non-reporting of the cases
encountered13. Quincke's edema
resolved within two days in the majority of cases even in our presented case.
The reason for unilateral tongue edema is not well understood; however, it is
assumed that unilateral edema precedes bilateral angioedema and that the
asymmetry of the lingual nerve results in a left-right difference in the
chemical microenvironment, resulting in unilateral sensitivity to the action of
inflammatory mediators12,14.
Recurrence can be observed if the treatment is not
stopped by error of diagnosis as in our case or as the case published by et all15, as it can recur even after stopping the
drug supposed to be in question (8) and this must review the diagnosis and
rediscuss the causal agent especially since the published cases of unilateral
angioedema of the tongue reveal more and more different causes such as
paracetamol, sartan, iodinated contrast agent, aspirin or recombinant
tissue-type plasminogen activator3,9,11,12,16.
Recurrence can be observed if the treatment is not
stopped by error of diagnosis as in our case or as the case published by
Mlynarek A, et al.15 as it can
recur even after stopping the drug supposed to be in question8 and this must review the diagnosis and
rediscuss the causal agent especially since the published cases of unilateral
angioedema of the tongue reveal more and more different causes such as
paracetamol, sartan, iodinated contrast agent, aspirin or recombinant
tissue-type plasminogen activator3,9,11,12,16.
Patients should be informed that angioedema can occur or reappear several weeks
after stopping treatment with ACE inhibitors. Although the incidence of
angioedema induced by ARBs II is much lower than that induced by ACE
inhibitors, cross-reactivity may occur in less than 10% of cases13. Therefore, the initiation of an ARB II
in patients who have experienced angioedema induced by ACE inhibitors should be
carefully evaluated based on the benefit/risk ratio.
Treatment of an ACE inhibitor-induced angioedema
attack is urgent because life is compromised, particularly if the edema is
significant and affects the upper respiratory tract. The basic treatment of ACE
inhibitor-induced angioedema consists of securing the upper airway and
preparing for artificial ventilation if necessary. Corticosteroids and
antihistamines are widely used in the management of these cases. Epinephrine
was also prescribed in our case, as in the case of Yuki Matsuhisa, et al.8.
The offending drug must be discontinued.
Although not yet approved by the U.S. Food and Drug
Administration, fresh frozen plasma (FFP) has been used successfully in some
patients. FFP contains ACE and some patients have reported marked improvement
after administration of two units17.
Although its efficacy remains controversial,
tranexamic acid (TXA) is used to treat hereditary angioedema caused by
inherited C1 esterase inhibitor deficiency in some countries.
One study evaluated TXA as an on-demand and
prophylactic treatment in patients with hereditary angioedema. The majority of
studies (80%) demonstrated the ineffectiveness of on-demand TXA for cutaneous,
abdominal or laryngeal swelling. In prophylaxis, approximately 50% of case
series, case reports and observational studies reported beneficial effects of
TXA18.
Newer therapies, such as icatibant and pdC1-INH, have
been shown to be more effective19.
Some studies consider prescribing ATX in cases of
recurrence of angioedema8.
However, there are no conventional recommendations on
the use of TXA in the treatment of drug-induced angioedema or in the prevention
of recurrence.
Conclusion
ACE inhibitor-induced angioedema is a potentially
serious adverse effect of ACE inhibitors and can be exacerbated by the use of
other medications (aspirin, paracetamol, iodinated contrast agents that
activate palsminogen, etc.).
Furthermore, unilateral angioedema of the tongue,
which is less frequently described in the literature, should raise this
diagnosis and, among other things, should consider the use of ACE inhibitors.
Recurrence after treatment discontinuation has also been observed, requiring
patient education. The mechanism of unilateral tongue involvement, as well as
that of recurrence after treatment discontinuation, remains unclear.
References
1. Hahn
J, Hoffmann TK, Bock B, Nordmann-Kleiner M, Trainotti S, Greve J. Angioedema.
Deutsches ärzteblatt international 2017( 114):489-496.
2. Bernstein
JA, Cremonesi P, Hoffmann TK, Hollingsworth J. Angioedema in the emergency
department: a practical guide to differential diagnosis and management. Int J
emergency Med. 2017;10(1):15-10.
3. Vedantam
V, Magacha HM, Vedantam N, Dahya V, Abu-Heija U. A Case Report of Losartan
Induced Angioedema. Cureus 2023;(3):15.
4. Reshef
A, Kidon M, Leibovich I. The Story of Angioedema: from Quincke to Bradykinin.
Clinical reviews in allergy and immunology 2016; (51):121-139
5. Banerji
A, Blumenthal KG, Lai KH, Zhou L. Epidemiology of ACE inhibitor angioedema
utilizing a large electronic health record. J allergy and clinical immunology
practice 2017;(3):744-749
6. Brown
T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced
angioedema: A review of the literature. J Clin hypertension 2017;(19):1377-1382.
7. Erickson
DL, Coop CA. Angiotensin-converting enzyme inhibitor-associated angioedema
treated with c1-esterase inhibitor: A case report and review of the literature.
Allergy rhinology providence 2016;(3):168-171
8. Matsuhisa Y, Kenzaka T, Shimizu H, Hirose H, Gotoh T.
Recurrence of unilateral angioedema of the tongue: A case report. World J Clin
cases 2023;11(27):6603-6612.
9. Anton
A, Jordan JF. Unilateral periocular angioedema after application of contrast
agent. Ophthalmologe 2015;112(10):854-856.
10. Park
SW, Bae IY, Eun HW, Park HW, Choe JW. Small-bowel angioedema during screening
computed tomography due to intravenous contrast material. J comput assisted
tomography 2011;35(5):549-552.
11. Deepthi
A, Kumar H, Ashraf S, Deepak JH. Images in Medicine - An Atypical Presentation
of Unilateral Tongue Angioedema Caused by Acetaminophen. Journal of Clinical
and Diagnostic Research for doctors 2017;11(4):ZJ01-ZJ02.
12. Kaptanoglu
AF, Aytas H. Aspirin-induced unilateral angioedema of the tongue. J European
academy dermatology and venereology 2006;(20):617-618.
13. Eloff
RP, Oosthuizen A. Unilateral tongue swelling: an unusual presentation of
angiotensin-converting enzyme inhibitor-related angioedema. BMJ Case Report
2024;17(8).
14. Leung
E, Hanna MY, Tehami N, Francombe J. Isolated unilateral tongue oedema: the
adverse effect of Angiotensin converting enzyme inhibitors. Current drug safety
2012;(7):382-383.
15. Mlynarek
A, Hagr A, Kost K. Angiotensin-converting enzyme inhibitor-induced unilateral
tongue angioedema. Otolaryngology head neck surgery 2003;(129):593-595.
16. Wollmach
AD, Zehnder D, Schwendinger M, Tarnutzer AA. Unilateral orolingual angioedema
in a patient with sarcoidosis after intravenous thrombolysis due to acute
stroke without improvement after treatment with icatibant. BMJ Case Report
2020;13.
17. Stewart
M, McGlone R. Fresh frozen plasma in the treatment of ACE inhibitor-induced
angioedema. BMJ Case Report 2012.
18. Horiuchi T, Hide M, Yamashita K, Ohsawa I. The use
of tranexamic acid for on-demand and prophylactic treatment of hereditary
angioedema-A systematic review journal of cutaneous immunology and allergy 2018.
19. Hide
M, Wang Y, Dote N, et al. Safety, efficacy and pharmacokinetics of icatibant
treatment in Japanese pediatric patients with hereditary angioedema: A phase 3,
open-label study. J dermatology 2023;50(11):1473-1477.