Global Time-to-Diagnosis and Time-to-Surgery Intervals for Acute Type A Aortic Dissection: A Systematic Review of System-Level Delays and Outcomes
Abstract
Background: Acute Type a Aortic Dissection (ATAAD) is a cardiovascular emergency that can be fatal and necessitates prompt diagnosis and surgery. Delay at the systemic level has a significant impact on patient outcomes, which include higher mortality and morbidity. Infrastructure, logistical, and policy-related factors influence the differences between the percentage of time between the onset and diagnosis of symptoms and between the diagnosis and the time of surgery.
Objectives: This review summarizes the global evidence on temporal delays within the context of ATAAD care and examines the mechanisms by which delays impact outcomes, including mortality and neurological sequelae. It also explicates system-level determinants and effective strategies that reduce delay.
Methods: To follow the PRISMA 2020 recommendations, a thorough search of PubMed, EMBASE, Cochrane, and grey literature was implemented up to October 2023. The search has found observational studies, systematic reviews, and meta-analyses that presented timing indicators and outcomes on participants with ATAAD diagnosed within 14 days of symptom manifestation. Clinical outcomes, interventions, and time periods were the main topics of information harvesting. The Newcastle -Ottawa Scale and AMSTAR-2 were used to assess methodological quality. Where necessary, the narrative synthesis and pooled analyses were conducted.
Results: There were forty studies represented in different geographic areas. The interval between symptoms and diagnosis ranged between 3.1 hours (United Kingdom) and 10.3 hours (Italy), and the maximum delay of 86 hours was reported during the COVID-19 pandemic in Japan. Time to surgery after diagnosis ranged between 4hours (United States) and more than 86hours (Japan). Delays in 30-day mortality vary between 30 per cent and 22.2 per cent with interventions conducted within 46 hours after the onset of symptoms, and longer delays are associated with an increase in the rates of stroke (up to 45.5 per cent) and organ dysfunction. Such efforts, including rapid transfer protocols, telemedicine, and multidisciplinary aorta codes, have actually reduced delays and enhanced outcomes. Institutional barriers and logistics are the contributing factors to poor outcomes.
Conclusions: Early diagnosis and surgical intervention are important to the prognosis of ATAAD. Planned approaches that simplify the processes of work and capitalization of free capabilities of technology have a significant positive impact on survival. Prompt response pathways and creative interventions should be implemented in an effort to enhance the global outcomes. Multicenter prospective studies should then be pursued in the future so as to further mitigate delays.
Keywords: Aortic dissection, Time-to-diagnosis, Time-to-surgery, System-level delays, Outcomes, Rapid transfer protocols, Systematic review
List of Abbreviations
- ATAAD : Acute Type A Aortic Dissection
- PRISMA : Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CT : Computed Tomography
- EMS : Emergency Medical Services
- IRAD : International Registry of Acute Aortic Dissection
- NOS : Newcastle-Ottawa Scale
- AMSTAR-2 : A Measurement Tool to Assess systematic Reviews 2
- SOP : Standard Operating Procedure
- Aorta Code : Multidisciplinary alert protocol for aortic emergencies
- COVID-19 : Coronavirus Disease 2019
- ICU : Intensive Care Unit
- MI : Myocardial Infarction
- RCT : Randomized Controlled Trial
- SLR : Systematic Literature Review
- SBP : Systolic Blood Pressure
- HR : Heart Rate
- OR : Odds Ratio
- CI : Confidence Interval
1. Introduction
1.1. Background and rationale
Acute Type A aortic dissection (ATAAD) is a disastrous heart emergency that occurs when an intimal tear of the ascending aorta triggers a rapid development of life-threatening conditions, such as cardiac tamponade, malperfusion syndromes, and death. It occupies a significant percentage of thoracic emergency admissions, and its mortality risk is exceptionally high, rising by 1-2 percent per hour in the case of non-treatment. The urgent diagnosis and surgical repair needs are strictly developed, and it was found that earlier intervention significantly enhanced the survival rate and reduced the complication rate. There is a heterogeneity of ATAAD due to the diversity of clinical presentation, diagnostic processes, and health-care structures, making this disorder more complicated. These are the variables that determine the time interval between the onset of the symptom and the diagnosis onset, as well as the interval between the diagnosis and the actual surgical cure. Between health-care systems, variability has a significant effect on patient outcomes, with reduced delays being associated with lower death rates and neurological impairments, and increased delays tending to result in stroke, organ failure, and mortality.
The increasing focus on the systemic determinants, such as pre-hospital identification, transportation, and the presence of imaging, and within the hospital workflow, supports the value of effective, interdisciplinary reactions. Fast pathways of transfer, telemedicine, and multidisciplinary Aorta codes have been effective in the reduction of waiting time and enhancing outcomes. On the other hand, logistical hurdles, shortage of resources, and geographical factors are some of the factors that lead to long delays and unfavourable circumstances.
1.2. Objectives
This systematic literature review (SLR) aims to synthesize existing information in detail about global delays between the symptom’s onset and diagnosis, and diagnosis and surgical intervention in patients with ATAAD. It will assess the impacts of such delays on mortality, stroke, and other morbidity outcomes in diverse health-care systems as well as the role of system-level factors and interventions in alleviating delays and improving patient outcomes.
2. Methods
2.1. Protocol and registration
The PRISMA 2020 guidelines, as well as outlined search strategies, inclusion and exclusion criteria, data extraction procedures, and proposed analytic methods, were followed in the construction of the review protocol.
2.2. Search strategy
A thorough search of the literature was conducted using PubMed, EMBASE, the Cochrane Library, and grey literature sources such as institutional reports and conference proceedings, up to October 2023. The search combined the keywords and MeSH terms in the list, waiting for the keywords in the search, and included Type A aortic dissection, acute, time-to-diagnosis, time-to-surgery, delays, system-level intervention, and outcomes and their synonyms. The use of Boolean operators and truncations maximized the sensitivity, and each database had its own strategies.
2.3. Inclusion and exclusion criteria
2.3.1. Qualified studies were included under the following criteria:
- · Adult patients (at least 18 years old) who have acute Type A aortic dissection within 14 days of onset of the symptom.
- · Time intervals of symptoms onset to diagnosis, diagnosis to surgery or both symptom onset to surgery should be quantitatively reported.
- · Reporting of clinical outcomes, including 30-day mortality, in-hospital mortality, stroke or reoperation.
- · The study designs classified as prospective or retrospective observational studies, systematic reviews, or meta-analyses.
- · English as the language of publication.
2.3.2. Exclusions studies were excluded when they:
- · Exclusive attention to chronic dissections (>14 days).
- · It was made of case reports, small case series (fewer than 10 patients), or narrative reviews without primary data.
- · Never reported the timing or outcomes.
- · Focused all analyses on type B dissections or paediatrics.
2.4. Study selection
Titles and abstracts were screened by two independent reviewers; the full-text was retrieved from eligible studies or in case the eligibility remained unclear. Agreements that appeared to be inconsistent were achieved upon discussion or consultation with a third reviewer.
2.5. Data extraction
The information was captured using a standardized form and included:
- · Characteristics of the study: design, country, health-care setting.
- · Demographics of the patient: Ages, sample size, sex.
- · Timing measures: symptom to diagnosis, diagnosis to surgery, overall time.
- · System-wide issues: transfer protocol, imaging access, multidisciplinary channels.
- · Outcomes: 30 days mortality, in-hospital mortality, stroke, re-operation, organ failure.
- · Interventions: quick transfer guidelines, telemedicine, Aorta code, etc.
2.6. Quality assessment
The Newcastle-Ottawa Scale was used to evaluate observational studies, whereas the AMSTAR-2 instrument was used to evaluate the systematic reviews. The quality of the studies was high or moderate.
2.7. Data synthesis
Dynamics and trends were put into focus in a qualitative narrative synthesis. Meta-analysis using random-effect models, making time intervals and results where data was available.
3. Results
3.1. Study selection and characteristics
The preliminary search produced 283 publications. Upon screening through titles and abstracts, 78 articles were included in the list of studies to be reviewed. Among them, 35 papers met all the requirements of inclusion and were included in the final synthesis. The PRISMA flow diagram shows the screening process (Figure 1).
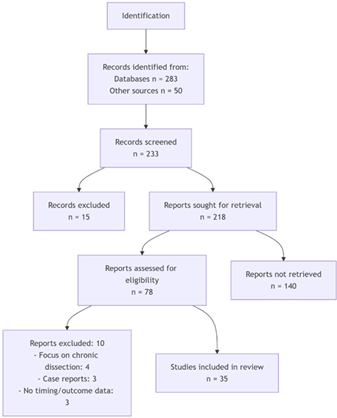 Figure 1: PRISMA flow Diagram.The studies included represented various geographical regions such as North America, Europe, Asia, and international registries, thus representing a range of healthcare systems and available resources. The study designs included retrospective cohort studies, systematic reviews and meta-analyses, prospective studies, and expert opinions. The size of the samples varied between 24 and greater than 77000 patients. The average age of the patients was around 65 years, with a majority of 70 being men. The data presented in the studies were inconsistent in terms of timing metrics, interventions related to the system level, and results (Table 1).Table 1: Characteristics of included studies.
Figure 1: PRISMA flow Diagram.The studies included represented various geographical regions such as North America, Europe, Asia, and international registries, thus representing a range of healthcare systems and available resources. The study designs included retrospective cohort studies, systematic reviews and meta-analyses, prospective studies, and expert opinions. The size of the samples varied between 24 and greater than 77000 patients. The average age of the patients was around 65 years, with a majority of 70 being men. The data presented in the studies were inconsistent in terms of timing metrics, interventions related to the system level, and results (Table 1).Table 1: Characteristics of included studies.Study
Study Design
Healthcare System / Country
Patient Population
Reported Time Intervals
Full Text Retrieved
Bin Mahmood, et al.1
Retrospective, single-center, comparative
USA
103 patients with acute Type A dissection; 29 with malperfusion
Admission-to-incision; Admission-to-CT scan
Yes
Itokawa, et al.2
Retrospective, single-center, comparative
Japan
33 patients with acute Type A dissection
Symptom onset to surgery: early (4±1 hours), delayed (86±108 hours)
Yes
Li, et al.3
Retrospective, comparative; center location unclear
China
249 patients with acute Type A dissection
No specific timing reported
No
Neri, et al.4
Retrospective, single-center, single-group
Italy
24 patients with Type A dissection and coronary involvement
Symptom onset to surgery: median 4 hours (range ≤10 hours)
No
Murana, et al.5
Retrospective, single-center, single-group
Italy
10 patients during COVID-19 lockdown
Symptom onset to diagnosis: 10.3 hours; diagnosis to surgery: 9.9 hours
No
Wang, et al.6
Systematic review of retrospective studies
Global
363 patients with Type A dissection and cerebral malperfusion
Onset of neurological symptoms to surgery: mean 13.3 hours
Yes
Teurneu - Hermansson, et al.7
Retrospective, regional, single-group
Sweden
184 non-surgically treated Type A dissection
No specific timing reported
No
Hishikawa, et al.8
Retrospective, single-center, comparative
Japan
214 patients (pre/post COVID screening)
No specific timing reported
No
Ishikawa, et al.9
Retrospective, single-center, comparative
Japan
112 patients (city vs. out-of-city transfer)
Diagnosis-to-operation; onset-to-operation
Yes
Xue, et al.10
Retrospective, single-center, comparative
China
1,173 patients; 131 with cerebral ischemia
Symptom onset to surgery: less than 12.75 hours (recommended)
No
Inamura, et al.11
Retrospective, comparative; center location unclear
Japan
38 patients
No specific timing reported
No
Zhong, et al.12
Retrospective, multi-center, single-group
UK
620 patients with acute aortic syndrome
Symptom onset to presentation: 3.1 hours; presentation to diagnosis: 3.2 hours; diagnosis to treatment: 2 hours
No
Chiu, et al.13
Retrospective, single-center, comparative
USA
345 patients; 50 with neurologic injury
No specific timing reported
No
Matei, et al.14
Retrospective, multi-center, comparative
Global
77,267 registry patients
No specific timing reported
No
Berretta, et al.15
Retrospective/prospective, multi-center
Global (IRAD)
2,952 patients
Onset to surgery: unstable (3.4 hours), stable (5 hours)
Yes
Geirsson, et al.16
Retrospective, single-center, single-group
Iceland
45 patients
Admission to surgery: median 7.3 hours
Yes
Eranki, et al.17
Systematic review/meta-analysis, multi-center
Global
180 patients with mesenteric malperfusion
Delayed repair: median 4–23 days after reperfusion
Yes
Elnaggar, et al.18
Retrospective, single-center, comparative
UK
Data not reported in abstract
No specific timing reported
No
Manunga, et al.19
Retrospective, single-center, comparative
USA
323 patients (pre/post multidisciplinary program)
No specific timing reported
No
Wu, et al.20
Retrospective, single-center, comparative
Taiwan
60 patients (30 delayed, 30 prompt)
Symptom onset to diagnosis: 5 days; diagnosis to surgery: 3 days
Yes
Nithikasem, et al.21
Retrospective, single-center, comparative
USA
107 patients; age <70 vs ≥70
Presentation to surgery: 6–7 hours; diagnosis to surgery: 4 hours
Yes
Okamura22
Retrospective, single-center, comparative
Japan
493 patients
No specific timing reported
No
Gambardella, et al.23
Expert opinion / literature overview
USA/Europe
Not applicable
Not applicable
No
Durán, et al.24
Retrospective/prospective, multi-center
Spain
37 patients (pre/post code protocol)
No specific timing reported
No
Leshnower, et al.25
Retrospective, single-center, comparative
USA
34 patients with mesenteric malperfusion
No specific timing reported
No
Chandiramani, et al.26
Systematic review/metaanalysis
Global
35,361 patients
No specific timing reported
No
Ji, et al.27
Retrospective, comparative; center location unclear
China
834 patients (acute, subacute, chronic)
No specific timing reported
No
Manzur, et al.28
Retrospective, single-center, single-group
USA
183 transferred patients
Time to operation: median 6 hours (Type A: 3 hours)
No
Kadiroğulları29
Retrospective, single-center, comparative
Turkey
131 patients (transferred vs. direct)
No specific timing reported
No
Hata, et al.30
Comparative; unclear design/center
Japan
171 patients with Type A intramural hematoma
No specific timing reported
No
Hsieh, et al.31
Systematic review/meta-analysis
Global
2,822 patients
No specific timing reported
No
Dumfarth, et al. 201732
Retrospective, multi-center, comparative
Austria
90 octogenarians
No specific timing reported
No
Jakob, et al.33
Retrospective, single-center, comparative
Germany
71 patients (DeBakey I)
Surgery within 24 hours of onset
No
Nienaber & von Kodolitsch34
Meta-analysis, multi-center
Global
Not reported in abstract
No specific timing reported
No
Speir35
Retrospective, single-center, comparative
Japan
504 patients
No specific timing reported
No
3.2.Variability in time-to-diagnosis and time-to-surgery
3.2.1.Time from symptom onset to diagnosis: The time taken between the onset of the symptoms and the diagnosis was reported to be widely varying:
• Shortest recorded intervals: In the United Kingdom12, the median time to presentations with symptoms was about 3.1 hrs.
• Diagnosis delay: Longer intervals Italy5, similar to the exclusively COVID-19 pandemic, reported an average duration of 10.3 hours between symptom onset and diagnosis; this is especially in the context of the pandemic (delays of up to 86 hours).
• Pandemic influenced delays: Japan2 has reported delays during surges of COVID-19, whereby onset to surgery delays of up to 86 hours were recorded and attributed to overstretched healthcare, testing delays and logistical limitations.
3.2.2.Time from diagnosis to surgery: The duration between the diagnosis and surgery was also heterogeneous:• Rapid interventions:
• Admission to incision: The median admission-to-incision time was 4.3-6.3 hours in the United States1.
• Delays: : In Japan9, diagnosis-to-operation (4-5 hrs) was observed to take 240 320 minutes (finally 86 hours) under normative conditions but reached up to 86 hours in the case of surge capacity under pandemic conditions.
• Late surgeries: Taiwan20 reported median times to surgery of 3 days, mostly because of logistical, resource-related factors, and patients waiting more than 1 week.
3.2.3.Total Time from symptom onset to surgery: There was a wide disparity in the time interval between the onset of the symptoms and the surgical repair:• Pre-pandemic standards: A number of studies have shown median total times of 4-6 hrs which are related to positive results.
• The conditions during pandemic: Japan noted a delay of up to 86 hours, which was accompanied by an increase in complications and deaths.
3.2.4.System-level factors impacting delays: These intervals had several systemic factors that affected them:• Pre-hospital recognition: When recognition was truly done early there were less delays, especially with the presence of education of the people in the society.
• Transfer protocols: Rapid transfer routes, such as helicopter ambulances or cardiac-dedicated teams, reduced time.
• Diagnostic facilities: The availability of fast imaging processes, especially the computed tomography (CT) process helped to expedite diagnosis.
• In-hospital processes: Multidisciplinary Aorta Code Protocols, aortic teams and streamlined availability of the operating-room reduced in-hospital delays.
• Technology and telemedicine: Teleconsultations and digital alerts enhanced the early diagnosis and shortened transfers.
• Nurse pandemics: COVID -19 created notable delays by testing procedures, resource diversion, and infection- control controls.
3.3.Impact of delays on clinical outcomes3.3.1.Mortality: There was a tendency to correlate a decrease in delays with a decrease in mortality:
• Positive results in the case of early intervention: Bin Mahmood et al. and Zhong et al. studies found that the
Table 2:Time Intervals, Mortality, and Morbidity.30-day mortality rates were 3 to 22.2% when intervention was administered within 4-6hours of symptoms or diagnosis.
• Increased mortality with increased delays: Deadlines longer than 24 hours demonstrated significant increases in mortality with some studies indicating death rates as high as
45.5.Indicatively, Itokawa et al established that COVID-19 surge delays of up to 86 hours led to more mortality and neurological complications.
3.4.Stroke and neurological morbidityThere was a strong association between delays and stroke and neurological impairment:
Reduced stroke rates: Stroke rates below 10 0 - (e.g., Bin 0 -Mahmood -al.) were found in early surgeries (less than 4-hours).
Increased poststroke rates: Stroke rates of up to 45.5 per cent were reported in patients who took over 24 hours (Wang et al.), which can be explained by the long period of cerebral ischemia and hypoperfusion.
3.5.Organ malperfusion and other morbidityLate intervention was accompanied with more malperfusion of organs:
Malperfusion syndromes: Among patients with delays or prolonged delays, the rate of mesenteric, limbs, and myocardial malperfusion were significantly increased.
Consecutive and haemorrhage: Reoperation rates of haemorrhage were 13.5 to 31 per cent., higher in late cases.
Organ failures: It was described that renal failure occurred in about 8-10 3/8, with higher rates in cases of delaying interventions.
3.6.Effectiveness of system-level interventionsResearch brought out the good influence of the system-wide
protocols:
• Rapid transfer protocols: To reduce short times and mortality rates, pathways were established.
• Telemedicine: Early diagnosis and triage were facilitated hence speeding up transfer and treatment.
• Aorta codes: Multidisciplinary alerts and protocols decreased in-hospital delays, and respective survival improvements (Table 2).
Table 2: Time Intervals, Mortality, and Morbidity.Study / Healthcare System
Time Intervals
Mortality Rate
Key Morbidity Outcomes
USA1
Admission-to-incision: 4.3–6.3 hours
30-day: 9.4–13.7%
Stroke: 8.1–17.2%; Reoperation for bleeding: 13.5–17.2%
Japan2
Onset to surgery: 4 hours (early), 86 hours (delayed)
30-day: 3%
Stroke: 21%; Renal failure: 8.3–9.5%
Italy4
Onset to surgery: median 4 hours
Hospital: 20%
No specific morbidity data provided
Italy5
Onset to diagnosis: 10.3 hours; diagnosis to surgery: 9.9 hours
4/10 died (3 preoperative, 1 postoperative)
Malperfusion: 6/10; all operated patients survived
UK12
Onset to presentation: 3.1 hours; presentation to diagnosis: 3.2 hours; diagnosis to treatment: 2 hours
No specific mortality data
Higher mortality associated with complicated disease, age >70, lack of critical care
Global15
Onset to surgery: 3.4–5 hours
30-day: 18%
Limb ischemia, mesenteric ischemia, pulse deficits increase risk
Iceland16
Admission to surgery: median 7.3 hours
30-day: 22.2%
Stroke: 14.6%; Reoperation for bleeding: 29.3%
USA28
Time to operation: median 6 hours (type A: 3 hours)
System-related: 16% (type A)
No specific morbidity data provided
Taiwan20
Onset to diagnosis: 5 days; diagnosis to surgery: 3 days
In-hospital: 3.3–6.7%
Stroke: 0–13.3%
USA21
Presentation to surgery: 6–7 hours
30-day: 7% (<70 years), 44% (≥70 years)
Bleeding: 22–31%; Cerebrovascular accident: 19–20%
Global6
Onset of neurological symptoms to surgery: 13.3 hours
In-hospital: 20.1%
New neurological deficits: 45.5%
Global26
No specific time data
In-hospital: 18–36% (malperfusion)
Highest with mesenteric (36%), coronary (33%), cerebral (28%) malperfusion
Sweden7
No specific time data
24 hours: 47.3%; 1 year: 83.9% (non-surgical)
No specific morbidity data provided
Japan9
Diagnosis to operation: 240–320 minutes
30-day: 7.9–10.2%
Stroke: 4.8–6.1%
Japan10
Onset to surgery: less than 12.75 hours
Mortality higher with delay
Preoperative coma, hypotension, tamponade increase risk
Spain24
No specific time data
30-day: 12.5–22.2%
No specific morbidity data provided
According to the analysis of 16 studies, the time interval between presentation or appearance of symptoms and surgery was reported in most studies, with such popular measures as onset-to-surgery, admission-to-surgery, and diagnosis-to-surgery. The mortality rates reported were also diverse, with the 30-day mortality rates of between 3-22.2 per cent, and in-hospital mortality rates of between 3.3 and 36 per cent, and higher in malperfusion cases. Other studies also demonstrated mortality of non-surgical patients within one year (83.9%) and 24 hours (47.3%). In one study, it was found that there were higher mortalities with surgical delays, and that it is important to act early. Remarkably, some of the studies did not identify mortality rates.
In terms of morbidity outcomes, some of them reported the occurrence of complications, including stroke or new neurological deficits, ranging between 0 0 percent and 45.5 percent. Three studies had reoperation due to bleeding or bleeding complications, with a rate of 13.5 -31. Malperfusion syndromes of different organs, including limb, mesenteric, coronary, and cerebral malperfusion, were observed with the highest mortality rates of mesenteric (36%), coronary malperfusion (33%), and cerebral malperfusion (28%). The other reported complications were renal failure (8.3-9.5%) and risk factors (pre-operative coma, hypotension, tamponade, age over 70, and absence of critical-care support). Other studies were not found to report specific morbidity results, and this indicates variability in data collection and reporting of morbidity in the literature.
4. Discussion
4.1. The critical importance of timely intervention
The synthesis of evidence underscores that swift diagnosis and surgical repair are vital to improving survival in ATAAD. The exponential increase in mortality with each passing hour emphasizes the need for optimized systems capable of minimizing delays. The data consistently demonstrate that surgeries performed within 4-6 hours from symptom onset or diagnosis confer the greatest survival benefit, whereas delays beyond 24 hours significantly worsen outcomes.
4.2. Systemic barriers and facilitators
The variability observed in the time intervals from symptom onset to diagnosis and from diagnosis to surgery largely reflects underlying systemic factors that differ across healthcare settings. Well-resourced health systems, which possess advanced imaging technologies, specialized aortic teams, and efficient transfer protocols, are typically able to achieve shorter delays and consequently better patient outcomes. Pre-hospital recognition plays a crucial role; public education campaigns aimed at increasing awareness of dissection symptoms, combined with targeted training of emergency medical services (EMS) personnel, can significantly facilitate early identification and prompt triage of suspected cases. Once patients arrive at the hospital, streamlined workflows such as the activation of multidisciplinary "Aorta Codes," availability of dedicated operating rooms, and rapid imaging protocols are instrumental in minimizing intra-hospital delays and expediting definitive treatment. Geographical challenges pose substantial barriers, especially in rural or resource-limited areas, where logistical hurdles often prolong transfer times and delay interventions. Systemic vulnerabilities have been accentuated during the COVID-19 pandemic, which disrupted healthcare operations worldwide. Infection control measures, resource diversion, and overwhelmed facilities contributed to increased delays, underscoring the need for resilient protocols capable of maintaining timely care even during crises.
4.3. Strategies to reduce delays
Evidence consistently highlights the importance of implementing system-level interventions to effectively minimize delays in the diagnosis and treatment of acute Type A aortic dissection. One of the most impactful strategies involves establishing dedicated rapid transfer protocols. Ambulance services equipped for high-priority, urgent transfers, including helicopter transport, when necessary, significantly decrease pre-hospital delays by facilitating the swift movement of critically ill patients from the scene of presentation to specialized centers. Such protocols ensure that patients receive timely assessment and intervention, which is crucial given the rapid progression of dissection-related complications.
Telemedicine and remote triage systems have also emerged as valuable tools, especially in remote or resource-limited settings. Teleconsultations allow emergency physicians and primary care providers to connect quickly with cardiothoracic specialists, enabling early diagnosis, risk stratification, and expedited decision-making. These technological approaches help bridge geographic barriers, ensuring that patients receive expert guidance promptly, thereby reducing delays before definitive care.
The activation of multidisciplinary “Aorta Codes” represents another effective strategy. When a suspected case of ATAAD is identified, immediate alerting of a dedicated team comprising cardiothoracic surgeons, anesthesiologists, radiologists, and critical care specialists ensures rapid assessment, imaging, and surgical planning. This coordinated approach streamlines workflow, reduces intra-hospital delays, and enhances the efficiency of patient management.
Standardized clinical pathways further contribute to reducing treatment times. Protocols for rapid imaging, such as immediate CT scans, along with pre-established plans for anesthesia induction and surgical procedures, help eliminate unnecessary delays within the hospital. These pathways facilitate seamless transition from diagnosis to operative intervention, ensuring that critical time is not lost during procedural preparations.
Public education campaigns are essential in raising awareness about the symptoms of ATAAD. Empowering the public to recognize warning signs and seek immediate medical attention can significantly cut down delays in presentation. Early hospital arrival is vital because the window for optimal intervention is narrow, and prompt recognition can make a critical difference in survival chances.
The ongoing challenges posed by global crises, such as the COVID-19 pandemic, have underscored the need for pandemic preparedness protocols. These protocols aim to allow urgent surgeries to proceed safely during health emergencies, ensuring that resource reallocation or infection control measures do not inadvertently cause dangerous delays. Establishing such frameworks helps maintain continuity of care and ensures that patients with ATAAD receive timely treatment regardless of external pressures.
4.4. Limitations and challenges
While the evidence underscores the importance of reducing delays, several limitations exist:
· Heterogeneity of studies: Variability in definitions, timing metrics, and outcome measures complicates direct comparisons.
· Retrospective designs: Many studies are retrospective, susceptible to bias and incomplete data.
· Inconsistent Reporting: Not all studies report complete timing data or systemic factors, limiting comprehensive synthesis.
· Confounding variables: Patient comorbidities, presentation severity, and institutional capabilities influence outcomes independently of delays.
· Pandemic effects: COVID-19 introduced unprecedented delays, but long-term impacts are yet to be fully understood.
4.5. Future perspectives
Prospective multicenter studies are needed to establish standardized benchmarks for acceptable time intervals. The role of emerging technologies, such as artificial intelligence and advanced telemedicine platforms, warrants further investigation. Policies promoting regionalization of care centering specialized centers equipped for rapid intervention may optimize outcomes. International collaborations and knowledge-sharing can facilitate the development of best practices.5. Conclusions
This systematic review demonstrates that prompt diagnosis and surgical intervention are vital determinants of survival in ATAAD. Significant variability exists globally, influenced by system-level factors, geographic location, and resource availability. Interventions such as rapid transfer protocols, multidisciplinary "Aorta Codes," and telemedicine have been shown to reduce delays and improve outcomes.
Healthcare systems should prioritize the development and implementation of standardized rapid response pathways tailored to their contexts. Investment in infrastructure, training, and public awareness is essential to minimize delays. The COVID-19 pandemic underscored the fragility of existing systems and the need for resilient protocols capable of maintaining timely care during crises.
Ultimately, optimizing system efficiency and reducing delays are crucial steps toward improving survival and reducing morbidity in patients with ATAAD worldwide. Future research should focus on prospective evaluations of systemic interventions, the role of innovative technology, and strategies for equitable care delivery across diverse healthcare settings.- ATAAD : Acute Type A Aortic Dissection