Automated Deployment in Action: A Case Study on IT Infrastructure Deployment with Ansible Playbooks
Larvicidal Activity of Sweet Wormwood (Artemisia annua L.) Extracts on Aedes aegypti (Tiger Mosquito)
Njila, H. L.1*, Hussaini, S. M.1, Valentine, C. C.1, Ombugadu, A.2, Polycarp, I. A.2, and John, A.D.3
1, Department of Science Laboratory Technology, University of Jos, P.M.B. 2084, Jos, Plateau State, Nigeria.
2. Department of Zoology, Faculty of Science, Federal University of Lafia, P.M.B. 146, Nasarawa State, Nigeria.
3. Department of Science and Technology, University of Jos, P.M.B. 2084, Jos,
Plateau State, Nigeria.
*Correspondence - Email: [email protected]; Telephone: +2348163365257
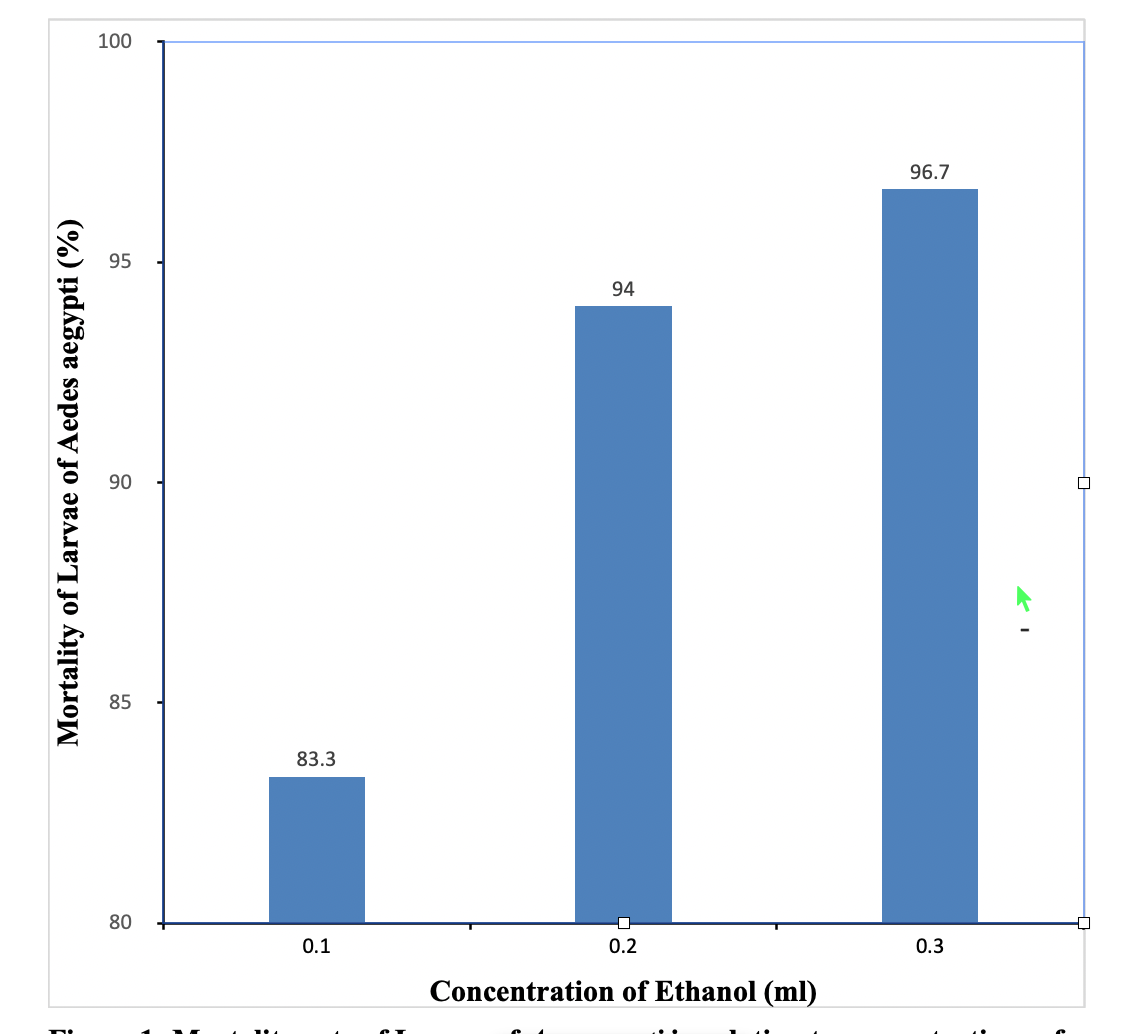
The control of mosquito using synthetic insecticides has some negative impacts such as environmental pollution and problem of resistance. Thus this work was aimed at evaluating the larvicidal activity of sweet wormwood (Artemisia annua L.) against Aedes aegypti mosquito. Hydrothermal extraction method was used to obtain leave extracts using 200 ml of ethanol, methanol and distilled water, respectively. Larvicidal bioassay was carried out using twenty five fourth instar larvae at 0.1 ml, 0.2 ml and 0.3 ml concentrations for each solvent extract. Set of control experiments using only the solvents were also set up. Mortalities were recorded from the first 15 minutes to 24 hours of exposure. The ethanol and methanol extracts of A. annua show a significant difference (P<0.05) in the mortality rates of Ae. aegypti larvae. However, there was no significant difference (P>0.05) in the mortality rates of Ae. aegypti larvae in relation to water extract of A. annua. The larvicidal activity of ethanol, methanol and water extracts of A. annua gave LC50 values of 0.131 ml, 0.602 ml and 0.25 ml respectively. The result of this study reveals that ethanol extract of A. annua is considered the best in terms of LC50 compared to methanol and water in causing mortality of the larvae. Thus it has great potential as a bio pesticides against Ae. aegypti.
Keywords: Larvicidal, Artemisia annua, Aedes aegypti, extract, Larvae
Introduction
Artemisia annua (Family-Asteraceae) has been used in traditional medicine for treating fever and malaria. There are several species of Artemisia known as aromatic fragrance plants that have a characteristic scent and taste [1]. The herb of Artemisia has been used medicinally to treat fevers for more than 2,000 years and to treat malaria for more than 1,000 years in China. Artemisia used in Chinese traditional medicine for centuries, is today considered part of the solution where malaria has become resistant to other medicines. Artemisinin-based combination therapies (ACTs) have been recommended by World Health Organization (WHO) since 2001 in all countries where falciparum malaria - the most resistant form of the disease - is endemic [2]. Zhang et al. [3] also proved that Artemisia showed the strongest biological activity in July, and found that the acaricidal activity varied significantly with the development of the individual plant. Mosquitoes have long been known for their importance as vectors of diseases [4]. Despite their small size, mosquitoes are of economic and medical importance. The genera Aedes, Anopheles, and Culex are important vectors of mosquito-borne diseases worldwide. Mosquito-borne diseases cause economic loss and are commonly found in tropical rather than temperate region [5]. Aedes aegypti, the primary carrier of the dengue virus, which is predominant in tropical regions, also transmits yellow fever in Africa and South America [6]. Cases of dengue fever and dengue hemorrhagic fever have increased every year and resulted in high number of deaths in Malaysia [7]. World Health Organization also reported that mosquitoes are one of the deadliest insects in the world. Their ability to carry and spread disease to humans causes millions of death every year. In 2015, malaria alone caused 438, 000 deaths. The worldwide incidence of dengue has risen 30-fold in the past 30 years, and more countries are reporting first outbreak of the disease. Zika, dengue, Chikungunya, yellow fever are all transmitted to humans by the Aedes aegypti mosquito, according to the World Health Organization [8]. Thus, this study was to evaluate the larvicidal activity of the leaves of sweet wormwood (Artemisia annua L.) on Aedes aegypti mosquito.
Materials and Methods
Study area
The larvicidal activities of sweet wormwood (Artemisia annua L.) on Aedes aegypti was carried out in the Insectary of the Department of Science Laboratory Technology, University of Jos, Nigeria (Latitude 09°57’ 01’’N and Longitude 08°53’ 21’’ E).
Collection of Plant Materials
The leaves of A. annua were collected from the Botanical Garden of the Department of Plant Science and Biotechnology, University of Jos, Nigeria.
Preparation of Plant Extracts
The collected leaves were dried at room temperature (27-37°C) for 10 -15 days. The dried leaves were crushed using electric blender. Twenty four grams (24 g) of the powdered leaves were orderly poured into three different dried and clean reagent bottles containing 200 ml of ethanol, distilled water and methanol, respectively. The samples were placed on a mechanical shaker (1000 rmm/min) for 3 hrs, after which the samples were left over night to settle and filtered the next day using funnel and filter paper to obtain the extracts. The extracts were then used to test for larvicidal activities on Aedes aegypti larvae.
Collection of Mosquito Larvae
Larvae of Ae. aegypti were collected from abandoned flower pots in Bauchi road campus of the University of Jos. The larvae were collected using standard dipping technique. The dipper was lowered at an angle of 450 to minimize disruption and the top of the water was skimmed so as to cause the nearby larvae to flow into the dipper. Care was taken not to spill water when raising the dipper from the water. However, if the flower pots have emerging vegetation, the water was disturbed so as to cause the larvae to swim downwards. Some of the vegetation were then removed using the dipper to create a clear spot for sampling. Larvae collected were transferred into a gallon for transportation to the insectary.
Larvicidal Bioassay
Concentration of 0.1 ml, 0.2 ml and 0.3 ml of extracts were tested. Two replications were done to ensure the validity of result. The larval mortality bioassay was carried out according to the test method for larval susceptibility proposed by the World Health Organization [9]. Twenty five fourth instar larvae of Ae. aegypti were placed in plastic bowls containing 40 ml of aqueous suspension of tested material at various concentrations. A set of control experiments using only the solvents were also set up. Mortality was recorded from first fifteen minutes to 24 hours of exposure and the larvae were not starved of food over this period.
Dose responses of larvicidal bioassay
The numbers of death larvae were counted from the first 15 minutes to 24 hours of exposure. The LC50 was calculated using probit analysis to find out the acute toxicity of the extracts.
Statistical Analyses
Data was analyzed using R Console software (Version 3.2.2). The observed mortality and proportions of observed mortality of Aedes aegypti larvae in relation to different concentrations of solvents used to extract the leaves of Artemisia annua were compared using