Recent Advancements In Sustainable Hydrogels for Sensing, Temperature Response, and Catalytic Applications
Abstract
Over
the previous few decades, the interest of chemists has shifted toward material
chemistry. The main reason behind this philosophical shift is the straitened
properties and dearth of wanted materials to fulfill the surfeited demand.
Material chemistry develops intellectual thoughts to manipulate the smart
materials of promising, desirable characteristics to suit further use.
Improvements from proffer solid to polymer and molecular to nanomaterial
unfurled its scope towards catalysts, sensors, molecular transporters, and
filters with the capability of immense scientific, social, and pecuniary
impact. Nowadays nanomaterials found profound exploitation at the industrial,
analytical, and domestic levels.
Keywords: Hydrogel, Optical
property, Sensing, Catalytic activity, Temperature response.
1.
Gel
A gel consists of two constituents, in which some cross-linkers
three-dimensionally interconnect the polymer chains. This structurally complex
3D network material (component 1) forms a rigid network, accommodating a
relatively large proportion of fluid (component 2). Both
inorganic and organic molecules can act as precursors for gelation. Depending
upon the composition of the gel, the forces of interaction for cross-linking
may differ from primary interaction to secondary interaction1-3. Gels are mushy materials Seemed solid
but are Susceptible to undergoing large contortion in response to external
stimuli4.
Polymer gel consists of a
three-dimensional network of elastic polymer chains including fluid between the
interstitial pores of the 3D network5.
Polymer gel exists in two phases swollen and collapsed, volume transition
between these phases can occur continuously or discontinuously6. Broadly polymer gel can be classified
into two classes based on dispersion medium, Aerogels, and Lyogels. In
aerogels, the dispersion medium is gas, while gels containing liquid as a
dispersion medium are known as lyogels7,8.
Lyogels containing non-polar liquid between their interstitial spaces are
called organo-gels, these gels tend to swell in the organic solvent. If
interstitial spaces are occupied by water, then they are known as hydrogels9.
Hydrogels are a 3D polymer network with a sufficient degree of
cross-linking, they are capable of absorbing and retaining a large amount of
water or aqueous solution (hundreds to thousands of times of its mass) without
dissolution10. Their degree of
flexibility is comparable to that of natural tissue due to their large
water-retaining capacity. The water-retaining proficiency of hydrogel arises
due to the hydrophilic functional group affixed to the polymeric backbone;
their resistance to dissolution is due to cross-linking between the polymer
chains11. Nonetheless, hydrogels contain large water content but
they show the characteristics of both solids and liquids, the transport of
small molecules and ions across the hydrogel by diffusion is analogous to
liquids, meanwhile at the macroscopic level, they exhibit the characteristics
of solids; preserve their shape and show mechanical strength like solids12. These Characteristics make them an
appropriate candidate for drug delivery, tissue engineering13, contact lens14, sensor15,
and catalysis16. A mesh is a
solid portion of a 3-D network of crosslinked polymer chains as shown in (Figure
1)17
Figure1:
Structural chemistry of a hydrogel,
“Hydrogels can be classified as follows”11.
2.1.
Hydrogels based on source
On the base of origin, hydrogels are
categorized into two groups. Natural hydrogels i.e. polysaccharides (like
hyaluronic acid, alginate, cellulose, and chitosan), proteins (e.g. collagen
and gelatin), DNA, etc3.
Synthetic hydrogels are fabricated by using various types of precursors (one or
more than one type)18. Poly
(NIPAM-co-2 HEA)19, poly (N,
N′-diethyl aminoethyl methacrylate), poly (N-isopropyl acrylamide)20, poly (N‐isopropyl acrylamide‐co‐vinyl
phosphonic acid)21 are some
examples of synthetic polymers.
2.2.
Hydrogels based on polymeric composition
On the base of the polymeric
composition, there are three classes of hydrogels.
2.2.1. Homopolymer gels: Those hydrogels which are synthesized by using
a single type of monomer are known as homopolymer hydrogels22. The Structural framework depends upon
the nature of the monomer, the nature of the cross-linker, and the type of
polymerization technique used for its fabrication. Polyethylene and
glycol-based hydrogels are examples of homopolymer gels. These types of
polymers contain only one type of monomer in their polymeric network, so their
properties are limited23.
2.2.2. Copolymer hydrogels: Hydrogels that are fabricated by using more
than one type of monomer are termed copolymeric hydrogels; usually, one type of
monomer is hydrophilic. Depending upon the type of precursors and reaction
conditions monomers can be arranged to form different configurations (i.e.
random, alternate, or block) to form a polymeric network24. Due to their multi-responsive nature,
they have several biomedical applications e.g. drug delivery25.
2.2.3. Multi polymer hydrogel: Multi-polymer hydrogels are fabricated by
using three or more types of monomers by polymerization technique and
cross-linking reaction. Poly (acrylic acid-2-hydroxy ethyl
methacrylate)/gelatin hydrogel is an example of a multi-polymer hydrogel. They
are multi-responsive due to the presence of more than one type of monomer26.
2.3.
Semi-interpenetrating polymeric hydrogels
In these types of hydrogels, one linear
polymer penetrates other cross-linked polymeric networks without forming any
other chemical bond between them. Due to the absence of an interpenetrating
network, they retain the rapid pH and temperature-responsive behavior, but they
can adjust their pore size and sluggish drug delivery. One example of this type
of gel is the entrapment of poly allyl-ammonium chloride in acrylamide /acrylic
acid copolymeric hydrogel. The resulting hydrogel contains both covalent as well
as ionic bonds, the covalent bond is responsible for the stability of the 3-D
network and the ionic bond is responsible for higher mechanical strength and
pH-responsive behavior27. These
types of gels have a large no of applications in drug delivery, for dyes, and
heavy metal separation28.
2.4.
Interpenetrating hydrogels
Such types of hydrogels are synthesized by intimate cross-linking
of two independent polymers, at least one component is the cross-linked polymer
and the other component is non-crosslinked11, This
is fabricated by immersing an already synthesized hydrogel into the solution of
the initiator and precursors. The interlocked cross-linked polymeric component
is responsible for its stability and morphology. The component polymer of such
hydrogels cannot be separated unless the chemical bond between them is broken29. The main advantage of this method is
that we can produce stiff and dense hydrogel metrics with desirable physical
and mechanical properties. The kinetics of drug delivery can be controlled by
tuning the pore size and surface morphology30.
2.5.
Classification based on physical appearance
Based on the physical appearance, there are three classes of
hydrogel, as shown in (Figure 2)31
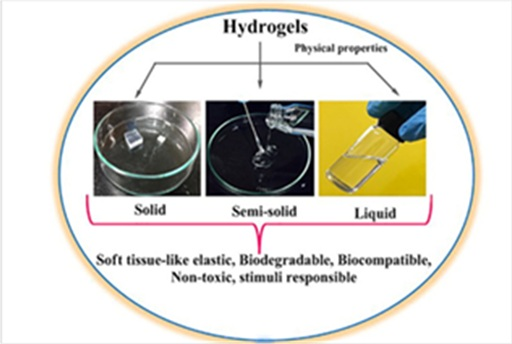
Figure
2: Physical properties of hydrogels.
2.5.1. Solid hydrogels: Those hydrogels that exist in a solid state at
room temperature are known as solid hydrogels. They can shrink and swell in
liquids: like water, biological fluids, and buffer solutions. Depending upon
the interest in using inorganic metal partials inside these types of hydrogels,
they can glow; which gives prominent electrical, magnetic, and optical
properties31.
2.5.2. Semi-solid hydrogels: These types of hydrogels possess strong
adhesive interaction with other types of interaction (hydrogen bonding, Vander
walls interactions, and electrostatic interaction) and a network of soft
tissues. Two different kinds of materials are used to fabricate them. One of
these must be natural like plant gum, poly (N vinyl Pyrrolidone),
Polycarbphhil, Carbopol, etc32.
The molecular weight of selecting polymeric material must be ≥ 100, which
enhances the flexibility of hydrogel. Due to bio-adhesive properties, these
hydrogels are named bio adhesives33.
2.5.3. Liquid hydrogels: Such hydrogels are in a liquid state at room
temperature, they exist as soft tissue-like elastic material at a specific
temperature, and they are easily fabricated34.
Due to their self-adjustment in their pore size, in response to the
environmental condition, they have a diverse number of applications: e.g.
organic and inorganic drug delivery processes. Due to their hydrophilic nature,
it is easy to incorporate protein inside them, so they are injected inside
living organisms for Vivo applications35.
2.6.
Classification based on type of cross-linking
There are three types of classes Based
on this classification, physically cross-linked, chemically cross-linked, and
Double-Network Hydrogels36.
2.6.1. Reversible or physically cross-linked hydrogels: In these types of
hydrogels, polymeric chains interconnect by molecular entanglement or secondary
forces: like hydrogen bonding or ionic interactions. The central role in
forming chemical interactions is hydrophobic interactions. Depending on the
environmental conditions such as pH and temperature, they can dissolve37,38. In
recent years, scientists have been interested in preparing physically
cross-linked hydrogels. The main advantage of this type of hydrogel is that
there is no need to use cross-linking agents. Cross-linking agents do not only
affect the integrity of hydrogels but some are sometimes also toxic, therefore,
must be removed before further use37.
2.6.2. Permanent or chemically cross-linked hydrogels: In these types of
hydrogels, the polymeric chains interconnect by a permanent covalent bond, so
they are unable to dissolve. They are stable39.
Depending upon the nature of functional groups present in the 3-D networks,
they can be charged or neutral. They may show a change in their shape in
response to the external electric field and other stimuli40. They have more mechanical Strength and
degradation time as compared to physically cross-linked hydrogels31.
2.6.3. Double-network hydrogels: In these types of hydrogels, there are two
types of cross-linking (both physical and chemical) are present. It overcomes
the disadvantages of using either physically cross-linked or chemically
cross-linked hydrogels. A recently reported graphene-polymer composite with
excellent mechanical properties and self-healing capability is an example of a
dual-network hydrogel37.
2.7.
Classification based upon stimuli response
Stimuli-responsive hydrogels show an unexpected change in growth action, mechanical strength, and structural network in response to external stimuli: so, they are known as Smart hydrogels, As shown in schematic (Figure 3)41 Light, Temperature, Pressure, mechanical stress, and intensity of various sources of energy are examples of physical stimuli.
Figure
3:
Stimuli-responsive behavior of hydrogels.
These stimuli are responsible for the change in the interaction
between the components of gels at specific critical points. Another type is
chemical stimuli, including pH, ionic factors, and chemical reagents: they are
responsible for the change in the interaction between the solvent and component
of gels and within gel at the molecular level42.
Many multi-responsive hydrogels are also reported, like a polyacrylic
acid-co-polyvinyl sulfonic acid-based hydrogel43.
2.7.1. pH-responsive hydrogels
Such polymeric gel, which contains the ionic
pendant groups, is pH-responsive. In response to the environmental conditions,
these groups pick and lose protons. They show a sudden dramatic change in their
ionization (pKa and pKb) at a specific pH, so they show
sudden volume change at a certain pH. This volume transition occurs due to the
generation of the electrostatic force of repulsion between the ionized groups,
which creates osmotic pressure for swellings. Both cationic and anionic
hydrogel show pH-responsive behavior. Anionic Hydrogels are made up of monomers
containing functional groups (like carboxylic, sulphonic, and phosphonic),
which donate protons above pKa, creating the same charge at
different sites within the hydrogel. But cationic hydrogels contain functional
groups like amines where protonation occurs below PCB; this leads to the
production of similar charges at different sites within the 3-D network, which
is responsible for volume transition44.
The degree of swelling is controlled by controlling the amount of cross-linker
during the preparation of hydrogel, ionic charge, pKa, or pKb
value of the ionizable groups, hydrophobicity, hydrophilicity, and degree of
ionization45.
2.7.2. Temperature-responsive hydrogels
Such
hydrogels that can shrink or swell in response to the temperature of
surrounding fluids are known as temperature-responsive hydrogels46. Chitosan-based copolymer hydrogels
extensively studied temperature-responsive hydrogels, like methylcellulose,
hydroxypropyl methylcellulose, and NIPAM. Poly (NIPAM) shows a sharp phase
transition at a temperature of 34.43 °C in water. P (NIPAM)’s LCT can be
altered by the copolymerization of other monomers. It can be increased by
copolymerization with hydrophilic monomers and can be decreased by
copolymerization with hydrophobic monomers. However, the grafting of such a
functional group does not have any appreciable effect on LCT. There are three
types of temperature-responsive hydrogels37.
2.7.2.1. Positive temperature hydrogels
They are known as upper
critical solution temperature (UCT) hydrogels. They are in a swell state at a
temperature above their UCT, but they exist in a shrinking state at a
temperature below their UCT. So as the temperature becomes below then the UCT
dehydration occurs and they release water from their interstitial pores. At
elevated temperatures, the breakage of hydrogen bonds occurs, so this creates
the same charge on different sites. As a result, the positive temperature
hydrogel exists in the maximum swelling state above its UCT20.
2.7.2.2. Negative temperature hydrogels
Such types of hydrogels
exist in the swell state, at a temperature below the LCT; and as the
temperature rises above its LCT, it shrinks. The most important parameter for
such types of hydrogels is LCT. LCT is controlled in no of ways: by changing
the solvent composition, by mixing a small amount of ionic copolymer, or by
changing the hydrophobic and hydrophilic cotenant polymeric hydrogels47. Their 3-D network contains both
hydrophobic and hydrophilic parts. At a temperature below the LCT, the
hydrophilic part interacts with water or fluid by forming a hydrogen bond. Due
to solvation, the swelling will increase. While at the temperature above the
LCT, the hydrophobic interaction between various parts of the hydrogel becomes
dominant; meanwhile, the hydrogen bonding becomes weaker. So, the hydrogel will
exist in shrink form at a temperature above its LCT.
2.7.2.3. Thermoreversible hydrogel
Such type of hydrogels has the same kind of
composition as that of positive temperature hydrogels and negative temperature
hydrogels. However, they differ in their bond type from the previous two types
of thermoresponsive hydrogels. Instead of covalently cross-linked polymer
chains that show volume change transition, they undergo a sole gel phase
transition. Different types of hydrophobic and hydrophilic interactions are
responsible for the abrupt change in their volume. In the swollen state, the
water molecules organize around the hydrophobic component of the gel (as in the
iceberg) by forming a hydrogen bond with the hydrophilic part of the gel. At
CST, the hydrophobic and hydrophilic interaction between polymer-polymer chains
and solvent-solvent becomes greater than the polymer-solvent interaction. So,
they exist in shrink form at CST37,48.
Hydrogels can be synthesized either from
natural or synthetic polymers. The chemical strength of synthetic polymers is
greater, and their degradation process is slow compared to natural polymers.
The greater durability of synthetic polymers is due to their greater mechanical
strength. These two properties are optimized during synthesis according to the
desired use. Their synthetic techniques are similar to those techniques, which
are used for the synthesis of cross-linked polymers. The schematic diagram for
the synthesis of hydrogel is shown in (Figure 4)11
Figure
4:
Schematic diagram of hydrogel preparation.
3.1. Bulk polymerization
It is the simplest method of synthesis,
in which one more type of monomer polymerizes to give the hydrogel of desired
properties. The type of initiators used depends upon the nature of monomers.
The rate of bulk polymerization is very high. There is a sharp increase in
viscosity as the reaction proceeds, and heat dissipation is a big problem of
this technique. These problems can be minimized, by controlling the rate of
polymerization. The end product of bulk polymerization is the glassy, hard, and
transparent homogenous gel. It swells to form soft and flexible materials when
immersed in water.
3.2. Solution polymerization
In solution polymerization, the mixture
of (neutral or ionic) monomers and cross-linkers is dissolved in a suitable
solvent. The redox initiator is used. The polymerization is initiated either
thermally or by irradiating UV light. After completion of the reaction: the
unreacted monomers, oligomers, initiator molecules, and other impurities are
removed by washing with distal water or DIW. The main advantage of this
technique over bulk polymerization is the use of a solvent that acts as a heat
sink. The most common solvents used in this technique are water, ethanol, a
mixture of water or ethanol, and benzyl alcohol. For the removal of the
synthetic solvent, the prepared gel may swell in water.
3.3. Suspension polymerization/Inverse
suspension polymerization
The end product of this method of
synthesis resulted in the form of powder or beads, so no further grinding is
required. “The process in which water-in-oil system (W/O) is used is termed an
inverse suspension polymerization, while in suspension polymerization process
oil-in-water (O/W) is used.” Suspension polymerization is a commonly used
technique. During this process, the homo genius mixture of monomers and
initiator molecules is dispersed in the organic phase. The addition of a
suspending agent and continuous agitation is necessary because dispersion is
not thermodynamically stable.
3.4. Suspension by irradiation
If we have to produce the hydrogel from
saturated compounds, the reaction is initiated by irradiating with high
energy/ionizing radiations like gamma rays or beams of electrons. This
irradiation is responsible for the production of free radicals on the polymer
chain and the radiolysis of water. The hydroxyl radicals (product of radiolysis
of water), and small fragments radicals (produced by the hemolytic cleavage of
a polymer chain) may attack the polymer chains to produce macro-free radicals.
These macro radicals may combine to produce a 3-D cross-linked network system.
The main superiority of this technique over the chemically initiated method is
the production of relatively pure and initiator-free hydrogels; i.e. poly
(vinyl alcohols), Poly (acrylic acid), and poly (ethylene glycol) are
cross-linked by this method.
4.
Nanotechnology
The concept of nanotechnology was given
by Nobel laureate Richard P. Feynman in his famous lecture “There are Plenty of
Rooms at the Bottom” in 1960. The word Nano originated from Latin and means
Dwarf. With the advancement in nanotechnology, a variety of materials is
synthesized. “Nanomaterial is such a substance which has at least one dimension
in the nanometer range’. There are many types of classification of nanomaterial49.
4.1.
Classification based on dimensionality
According to this type of
classification; basically, there are four types of nanomaterial: i.e. 0D, 1D,
2D, and 3D. Dimensionality is generally represented by the notation “kD/mn”.
The value of k represents the dimensionality of overall materials, while the
value of l, m, and n designates the dimensionality of the building blocks of
the overall material. The value of k must be equal to or greater than the value
of l, m, and n. According to this type of class, there are 36 types of
nanomaterials50.
4.2.
Classification based upon composition
There is another type of classification,
“which is according to the chemical composition of constituents51."
4.2.1. Metal-based nanomaterials
Metal-based nanomaterials may be pure metal or metal alloys51. Silver-based nanomaterials have
catalytic, diagnostic, conductive, antibacterial, and optical applications.
Copper-based partials and their assemblies are used, as catalysts, electrical
conductors, thermal conductors, antibacterial agents, and lubricant additives51.
The gold-based nanostructure is exploited for drug delivery systems and
specialized alloys, medical testing and cancer detections, dissipation of heat,
and fuel cells. Due to their low density and high heat resistance, the
nanostructures based on Al-Mg alloy, Mg, Al, and titanium are employed in
aerospace technology52. Because
of its unique magnetic properties, the Fe-Si-B alloy is used for the
fabrication of electronic devices53.
Iron, Platinum, and palladium nanoparticles are used as catalysts54.
4.2.2. Metal oxide-based nanomaterials
Titanium oxide, cerium
oxide, and zinc oxide nanoparticles are used as sun screeners in cosmetics
products due to filter UV radiation. Nano-silica has good optical absorption
ability and photoluminescence and is also used in drug delivery systems. Nano alumina
coating acts as an abrasion resister acts as a catalyst and is termly
conductive. Depending on the nature of iron oxide, it might be used in the
biomedical field, as an ion exchanger, or in the fabrication of electronic
structures. The iron oxide nanomaterials may be employed as pigments or might
be for the purification of water55-57.
4.2.3. Semiconductor nanomaterials
At the nanoscale, the properties of materials
are owned by quantum mechanics58.
Nano silicon has applications in solar cells and the biological field and
photovoltaic etc. gallium nitride and gallium arsenide at nanoscale use in
photovoltaic and in the production of light-emitting diodes.
4.2.4. Nanomaterial including silicates, carbonates, and nitrates
Clay (a nano silicate)
can take the shape of a nanotube or nanoplates. Clay may be employed as filler
in composites to enhance its mechanical properties, improve electrical
conductance, and increase resistance against heat or flame. Nanoparticles of
silicon nitrides might be used to decrease the possibility of propagation of
the crack. Zeolite at the nanoscale has catalytic applications and may be used
in membrane fabrications59.
4.2.5. Carbon-based nanomaterials
Carbon-based nanomaterials represent one of
the best classes of nanomaterials. Graphene is a one-atom-thick sheet made up
of carbon. It has promising applications but its industrial synthesis is under
development60. Grephen’s
self-alignment in cylindrical form due to wander wall forces from carbon
nanotubes61. Carbon nanofibers
are also folded graphene but without perfect arrangements. Fullerenes are
spherical, formed by the folding of graphene. Carbon black is amorphous carbon
particles left as a byproduct of incomplete combustion62,63.
4.2.5.1. Nano polymers: Nano capsules and nanospheres are examples of
nano polymers. They have an important use in drug delivery64.
4.2.5.2. Dendrimers: There are two parts of dendrimers, one is an
internal core and the other is the outer branch. The branches can be modified
according to requirements. They are important candidates for drug delivery65.
4.3.
Classification based upon origin
There are two types of nanomaterials, synthetic and natural.
Naturally occurring nanomaterials are either synthesized within living bodies
or by anthropogenic actions outside the bodies. They are found everywhere:
inside the earth's crust, in oceans, on the earth's surface, or in the
atmosphere. Human DNA is an excellent example of naturally occurring
nanomaterials. Numerous varieties of nanomaterials are synthesized in
laboratories and on a commercial scale66.
There are two techniques adopted for the synthesis of
nanoparticles, “termed as bottom-up method and top to the down method,” as
shown in schematic (Figure 5)67
A relatively new technique available only in modern laboratories,” the Bottom
to Bottom” approach is also adopted51.
Figure
5:
Schematic diagram of top-down and bottom-up synthesis methods.
5.1.
Top-down method
In the "top-down method," we
start with a substance in bulk form and end with nanoscale objects by applying
either mechanical stress (grinding, milling, or etching); or by using some
chemical agents. The most popular physical method for the top-down approach is
lithography, and the other relatively new is electric erosion68. It's an easy and cheaper method, but it
has many shortcomings; the end product is polydisperse and may have defects in
its morphology51.
5.2.
Bottom up method
In this approach of fabrication, we
start with atoms or molecules and end with nano-scale objects. During this
technique, the first step is the formation of stable nuclei, and the next step
is growth. By controlling the growth, we can control the overall size of the
resulting nano object. By controlling the growth at different sites, we can
control the morphology of results from materials69.
A microgel containing metal particles inside their interstitial
pores is known as a hybrid microgel. The schematic diagram for the synthesis of
hybrid microgel is shown in (Figure 1.6). The nanoparticle is the
aggregation of bounded atoms having a three-dimensional radius within the range
of a nanometer. The nanoparticles are highly reactive, having a high
surface-to-volume ratio and high surface energy. Due to high interfacial activities,
they tend to react with contaminants in water, gas, and the earth. So, they are
beneficial candidates for controlling environmental pollution. Due to their
unstable nature, they have great difficulties in their mass transport. Their
separation, reusability, and prevention from agglomeration are difficult tasks.
They may be risky for human health and the ecosystem. These risks and
difficulties can be minimized; by their incorporation into the gel or other
porous material70. Such hybrid
gels are known as smart materials due to their stimuli-responsive nature71.
Figure 6:
Hybrid microgel enhanced physical and chemical properties.
6.1. Responsive hybrid microgels
The hybrid gel may respond to different
stimuli (like pH and temperature) depending on its chemical composition. The
partial size may vary in response to environmental conditions72.
6.1.1. Temperature-responsive hybrid
microgels
Depending upon the
composition of the microgel, their hydrodynamic volume may increase or decrease
with the change of temperature of the medium. The most studied
temperature-responsive hybrid gels are poly (NIPAM) and PVC-based. The
temperature at which the hybrid system shows abrupt change is known as the VPTT
of that hybrid system. NIPAM-based microgel shows a decrease in hydrodynamic
radius with an increase in medium temperature.
6.1.2. Glucose-responsive hybrid microgels
Sliver NP’s incorporated hybrid gel
containing Phenyl boric acid in their host polymeric gel is glucose-responsive.
Poly (3-Acrylamidephenylboronic-co-acrylic acid)-Ag NPs show an increase in
hydrodynamic volume in response to a rise in Glucose concentration of the
medium73.
6.1.3. pH-responsive hybrid microgels
Such hybrid gel shows the change in
hydrodynamic radius in response to the alteration of the medium’s pH. Such a
system contains ionizable functional groups in the polymeric gel. The hybrid
gels containing amino groups in their 3-D polymeric network exhibit an increase
in volume due to the protonation of the amino group. So, they exist in a
swollen state when the pH of the medium is high. Gels containing carboxylic
groups in their 3-D network show an increase in volume at pH ≥ PKA of ionizing
species.
6.1.4. Ionic-responsive hybrid microgels
Such hybrid gels respond to the change
in the concentration of ions in the medium. They are responsive to different
electrolytes like KCl and CdCl2. Investigation shows that VPTT moves
towards the lower temperature, as the salt concentration of the medium
increases.
6.1.5. Multi-responsive hybrid microgel
Those hybrid gels, which can respond to
more than one stimulus, are known as multi-responsive hybrid gels, as shown in (Figure
7).
Figure
7:
Multi-responsive behavior of silver-embedded microgels towards external
stimuli.
They contain more than one type of monomer in their 3-D structure.
For example, Ag NPs loaded poly (N-isopropyl acrylamide-co-acrylamide-co-vinyl
phenylboronic acid) gel is pH, Glucose concentration, and
temperature-responsive. Poly (N-isopropyl acrylamide-co-vinylphenylboronic
acid), and poly (acrylamide-co-vinylphenylboronic acid) show sensitivity toward
glucose concentration, temperature, and pH. At a given pH of the medium, they
show increases in volume with the increase in the concentration of the medium,
because more glucose moves inside.
7. In situ synthesis of metal nano
particles
For "in situ synthesis," the ions of metals are loaded inside porous material; pours material is a gel for hybrid gel synthesis. Then the calculated amount of some reducing agent is added for the synthesis of zero-valent metal nanoparticles. The schematic diagram for the Situ synthesis of silver particles is shown in (Figure 8)74 Such a metal ion-loaded system may interact with Se2-, OH-1, or S2- depending upon the required composition of the metal nanoparticle75,76.
Figure
8:
Fabrication of Silver nanoparticles inside microgel by in-situ reduction
method.
Other methodologies of in situ synthesis are mixing the
nanoparticle and precursor of the host polymeric system. Then the
polymerization is preceded by the desired condition to make a hybrid system77. The other approach is mixing the
precursor of both “metal nanoparticles and host polymer” in a suitable solvent78.
Silver gives a white and lustrous appearance; these soft
transition elements possess excellent catalytic and thermal properties. Silver
nanoparticles may be employed as a sensor and as antibacterial agents79. They tend to agglomerate because of high
interfacial activity. "Microgel provides stability to silver particles due
to different anchoring functional groups; moreover, we can tune the size of the
particle inside the gel80."
8.1. Applications of silver embedded
hybrid microgels
8.1.1. Catalytic applications:
Because of the high surface-to-volume ratio, the silver NPs are an efficient
catalyst. However, due to their high interfacial activities, smaller NPs
aggregate to form bigger partials, so as a result, their catalytic activity
decreases. Many substances like dendrimers, surfactants, and polymer brushes
are used as a stabilizer for Ag NPs. The silver NPs stabilized by these
substances cannot be reused. Moreover, there is a greater chance of poisoning
and contamination of catalysts during the catalytic reaction. The Ag NP’s
embedded microgel is a very impressive microreactor for catalysis. Their
catalytic activity is many times faster than other systems. The inert polymeric
network eases the diffusion of reactants toward the Ag NP’s surface and allows
the outward diffusions of the product. Their catalytic activity can be tuned by
changing external stimuli.
8.1.2. Biomedical applications:
Some stimuli-responsive Ag NPs encapsulated hydrogels are used for blood
glucose detection and controlled delivery of insulin. The fluorescence
intensity of some biomolecules is very low, so there is a problem with their
detection by fluorescence analysis. The coupling of such molecules with Ag NP’s
loaded microgel can enhance their fluorescence intensity. The detection of
porphyrin by metal-enhanced fluorescence effect is reported in the literature.
It is used for cell imaging.
8.1.3. Applications in semiconductor
devices:
When Ag NP’s embedded hybrid gel is encapsulated in a hydrophobic shell, a such
hybrid gel is no more sensitive to stimuli except light. Hybrid gel exists in a
collapsed state at high temperatures, so the distance between the NPs
decreased. When light is irradiated, the reflectance increases because there
are no spaces through which light can pass. Their suspension may be used as
photonic crystals in different fields.
8.1.4.
Calorimetric sensing: In clinical
procedures, pharmaceutical industries, food industries, and environmental
analysis, hydrogen peroxide determination is very important81. Several methodologies like the
chromatographic technique82,
titrimetric analysis83, chemiluminescenc84, fluorometry85,
Spectrophotometric analysis86,
and electrochemistry87 are used
for (hydrogen peroxide) analysis. These conventional methods are usually
time-consuming, difficult, and complicated. With the advancement of
nanotechnology, the most commonly used electrochemistry technique is improved
by replacing enzyme-based biosensors with nanoparticle-based sensors. Because
of efficient catalysis, excellent surface-enhanced Raman scattering, low
toxicity, and biocompatibility the Ag NPs earned significant fame in hydrogen
peroxide sensing88,89. The silver-embedded three-dimensional
network system is an efficient and cheap candidate for hydrogen peroxide
sensing90.
8.1.5. Optical properties: In response to external stimuli, the hybrid
gel can swell and de-swell. As a result, a change in surrounding the effect of
solvent, refractive index of solvent, interparticle distance of embedded
particle distance, and surface effect changes. So, by changing the condition of
the surrounding medium, we can change the surface Plasmon band91.
9. Conclusion
Recent advancements in sustainable hydrogels have significantly
expanded their potential in sensing, temperature-responsive systems, and
catalytic applications. Innovations in biodegradable polymers, bio-based
materials, and green synthesis methods have improved hydrogel performance while
minimizing environmental impact. These materials now exhibit enhanced
sensitivity, tunable responsiveness, and improved catalytic efficiency, making
them highly attractive for diverse applications, including biosensing, drug
delivery, and environmental remediation. Despite these achievements, challenges
such as scalability, long-term stability, and cost-effective production remain.
Future research should optimize hydrogel properties through advanced material
design and integrate them with emerging technologies such as nanomaterials and
smart devices. By addressing these challenges, sustainable hydrogels will
continue to play a crucial role in developing eco-friendly and high-performance
functional materials.
10. Acknowledgment
We Acknowledge this article to a brave leader (Imran Khan) of
Pakistan.
11. Declaration of competing interest
The authors declare that they have no known competing financial
interests or personal relationships that could have appeared to influence the
work reported in this paper.
12. Data availability
Data will be made available on request.
13. References
1.Memon IA, Xudong L, Xiaoqing C, et al. A review of
electro-stimulated gels and their applications: Present state and future
perspectives, in: J Mater Sci, 2019: 109852.
4.Osada Y, Gong JP.
Soft and wet materials: polymer gels, Adv. Mater, 1998;10: 827-837.
5. Ahn SK, Kasi RM,
Kim SC, et al. Stimuli-responsive polymer gels. Soft Matter, 2008;4: 1151-1157.
6. Annaka M, Tanaka T. Multiple phases of polymer
gels. Nature, 1992;355: 430-432.
9.Nabi SAA, Sheraz MA, Ahmed S, et al. Pharmaceutical
Gels: A Review. J Pharm. Pharm Sci, 2016;4: 40-48.
17. Gibas I, Janik H.
Synthetic polymer hydrogels for biomedical applications, 2010.
18.Gyles DA, Castro LD, Silva Jr JOC, et al. A review of
the designs and prominent biomedical advances of natural and synthetic hydrogel
formulations. Eur Polym J, 2017;88: 373-392.
20.Qizu Y, Park K.
Environment-sensitive hydrogels for drug delivery. J Mater Sci, 2001;53:
321-339.
30. de Britto D,
Campana-Filho SP. Kinetics of the thermal degradation of chitosan.
Thermochimica acta, 2007;465: 73-82.
46. Richter A.
Hydrogels for actuators, in: Hydrogel sensors and actuators. Springer, 2009:
221-248.
52. Morris DG. The
origins of strengthening in nanostructured metals and alloys, 2010.
56. Dioxide NT. State
of the Science Literature Review: Nano Titanium Dioxide Environmental Matters.
64. Rangasamy M. Nano
technology: a review. J Appl Pharm Sci, 2011;1: 8-16.
65. Khopde A. Dendrimer as a potential delivery system for
bioactive In: Jain NK, editor. Advances in controlled and novel drug delivery.
CBS publisher, 2001.
68. Ramsden JJ.
Nanotechnology: an Introduction. William Andrew, 2016.
70. Zhao X, Lv L, Pan B, et al. Polymer-supported
nanocomposites for environmental application: a review. Chem Eng J, 2011;170:
381-394.
71. Zhang Y, Gu W, Xu H, et al. Facile fabrication of hybrid
nanoparticles surface grafted with multi‐responsive polymer brushes via block
copolymer micellization and self‐catalyzed core gelation. J Appl Polym Sci,
2008;46: 2379-2389.
72.Liu YY, Liu XY, Yang JM, et al. Investigation of Ag
nanoparticles loading temperature responsive hybrid microgels and their
temperature controlled catalytic activity. Colloids Surf A Physicochem. Eng
Asp, 2012;393: 105-110.
74.Begum R, Naseem K, Farooqi ZH. A review of responsive
hybrid microgels fabricated with silver nanoparticles: synthesis,
classification, characterization and applications. J Solgel Sci Technol,
2016;77: 497-515.
84.Lei W, Dürkop
A, Lin Z, et al. Detection of hydrogen peroxide in river water via a
microplate luminescence assay with time-resolved (“gated”) detection. Mikrochim
Acta, 2003;143: 269-274.