Topical Tacrolimus-Induced Hyperpigmentation: A Rare Adverse Drug Reaction Worth Reporting
Abstract
Background
Hyperpigmentation is a common dermatological condition characterized by
darkening of the skin due to increased melanin synthesis or altered melanin
distribution. Drug-induced pigmentation (DIP) accounts for approximately 20% of
acquired hyperpigmentation cases, with over 50 implicated drugs, including
chemotherapeutic agents, antibiotics and immunosuppressants. Tacrolimus, a
calcineurin inhibitor used in atopic dermatitis, is rarely associated with
hyperpigmentation.
Case presentation
A 22-year-old female presented with perioral eczema
unresponsive to topical corticosteroids. She was prescribed tacrolimus 0.1%
ointment for one month. After two weeks, she developed gradual darkening of her
lips, with no associated itching, burning or edema. There was hyperpigmentation
at the angles of the mouth without ulceration or scaling. She had no history of
smoking, sun exposure or other drug use. Tacrolimus was discontinued and
sunscreen with mild moisturizer was advised, leading to partial resolution of
pigmentation within two months. Causality assessment using the Naranjo scale
yielded a score of 6, indicating a probable relationship between tacrolimus and
the adverse reaction.
Discussion
Hyperpigmentation due to topical tacrolimus is rare. The proposed mechanisms
include increased melanin production secondary to mast cell activation or
melanocyte stimulation. Photo-protection and early drug withdrawal are
essential to prevent cosmetic complications.
Conclusion
This case highlights hyperpigmentation as an uncommon but noteworthy adverse
drug reaction to topical tacrolimus. Clinicians should recognize and report
such rare events to strengthen pharmacovigilance and enhance understanding of
tacrolimus-related cutaneous adverse effects.
Keywords: Tacrolimus,
Atopic dermatitis, Skin hyperpigmentation, Adverse drug reaction
1.
Introduction
Hyperpigmentation of the skin is a common
dermatological condition in which the color of the skin generally becomes
darker. A common dermatological disorder known as hyperpigmentation causes the
skin to become darker overall. Numerous internal and external variables, such
as hormone fluctuations, inflammation, trauma, acne, eczema, certain
medications, UV exposure, etc., can cause these colour changes in the skin. The
biological mechanisms involving the synthesis of melanin, the skin pigment, by
melanocytes in the different layers of skin control skin pigmentation and coloration.
Skin hyperpigmentation diseases are thus caused by changes in melanocyte
production or melanin dispersion1.
Among the many causes the most common cause of skin hyperpigmentation is
genetics, exposure to sun and drug induced2.
An estimated 20% of all cases of acquired
hyperpigmentation are thought to be caused by drug-induced pigmentation (DIP).
Antibiotics, antimalarials, antiretrovirals, antipsychotics, prostaglandin
analogues, heavy metals and chemotherapeutic drugs are among the more than 50
substances that have been linked. The colour, location, onset and duration of
pigmentation differ amongst offending chemicals and they can impact the skin,
mucosal surfaces, nails and hair. Amongst these drugs,
chemotherapeutic agents are a very likely cause of DIP3. In this case, the patient used
Tacrolimus for atopic dermatitis which led to skin hyperpigmentation. Usually,
the first choice of treatment for this condition is corticosteroids, however, Tacrolimus
ointment is an alternative treatment4.
Tacrolimus being a calcineurin inhibitor it blocks the
activation of T cells and production of inflammatory cytokines5. Both oral and topical formulation are
available. The topical formulation is used in eczema and other inflammatory
dermatological conditions and also an immunosuppressive agent for prophylaxis
of organ rejection. Systemic usage of tacrolimus has adverse effects of
nephrotoxicity, neurotoxicity, diabetes and other metabolic imbalances.
Dermatological adverse reactions like hyperpigmentation on usage of topical
tacrolimus are acne vulgaris, alopecia and rash but these are rarely seen6. According to the Vigiaccess
database, skin hyperpigmentation was seen in 33 cases out of more than 96,000
reported ADR’s (Adverse Drug Reaction)7.
This makes it a very rare adverse event and hence is worth reporting.
2.
Case Report
This case was collected as a part of the
Pharmacovigilance elective under the Department of Pharmacology, Christian
Medical College, Ludhiana, which is an ADR Monitoring Centre. A 22-year-old
female patient presented to the Dermatology OPD with history of eczema on the
lips and perioral region and was initially treated with topical steroids for 1
week. Since the issue didn’t subside with topical steroids, she was prescribed
with tacrolimus ointment 0.1 percent for 1month HS. After 2 weeks of
application of the ointment, she noticed gradual darkening of her lips. With
progressive pigmentation, she was worried about the cosmetic effect. The
patient was a non-smoker without a history of hyper exposure to the sun, drug
exposure or other etiological conditions for lip hyperpigmentation. There were
no systemic symptoms of itching, burning, edema. There was hyperpigmentation at
the angle of mouth without any scaling or ulceration. Tacrolimus was stopped
and sunscreen and mild moisturizer were advised. The pigmentation decreased to
a certain degree within 2 months. This is shown in (Figure 1).
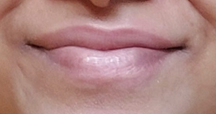

a) 1 week after
patient started with Tacrolimus b) 2
months after stopping the treatment with Tacrolimus
Figure 1:
Shows skin hyperpigmentation after starting and stopping tacrolimus
The Naranjo score was 6[probable] and hence, causality
assessment showed a probable correlation with the current adverse effect.
The standardized, structured Naranjo Probability
Scale, which is used to assess the likelihood that a specific medicine is the
cause of an adverse event, consists of ten components. Each question is
assigned a score based on the response- “Yes,” “No,” or “Do not know”-with
corresponding point values of -1, 0, +1 or +2, depending on the direction and
intensity of the evidence. Based on the cumulative score, the likelihood of an
ADR is categorized as questionable (score ≤0),
plausible (score 5–8), possible (score 1–4) or definite (score ≥9). The overall score, which indicates the extent
of causality, ranges from -4 to +138.
3.
Discussion
World Health Organization defines ADR as “a response
to a medication that is noxious and unintended and occurs at doses normally
used in man9.” Patients'
quality of life may be significantly impacted by ADRs, which can also put more
strain on the healthcare system. ADRs are one of the leading causes of
morbidity and mortality on a global scale and they continue to pose a serious
health concern if not identified and tackled diligently10. Tacrolimus, a calcineurin
inhibitor, is an immunosuppressive drug used in organ rejection prophylaxis
after organ transplantation and for autoimmune conditions like atopic
dermatitis and is of the rare causes of DIP3,5.
Identification of drug causing DIP requires a
comprehensive physical examination and medication history are required. The
most common mechanism of DIP is accumulation of melanin which is either by a
direct trigger of the medication or nonspecific inflammation caused by the drug.
A key factor in preventing DIP aggravation is photo-protection, which includes
wearing photo-protective clothes, applying a broad-spectrum sunscreen and
finding shade. DIP clearance has been achieved using a number of lasers,
including the picosecond alexandrite, Q-switched ruby, Q-switched alexandrite
and Q-switched Nd:YAG lasers3.
Hyperpigmentation is an unusual ADR of tacrolimus, with
isolated reports. The mechanism of this effect is unclear, but theories include
enhanced melanin production secondary to tacrolimus induced mast cell
activation, melanocyte stimulation or phototoxicity. In systemic tacrolimus, hyperpigmentation
in transplant patients manifests as an effect on the face and the nails. In
topical tacrolimus, events are far less frequent11.
The following pie chart shows the distribution of ADRs
related to tacrolimus affecting different body systems7. This is shown in (Figure 2).
The ‘’others’’ category involves categories with less than 1% reported cases.
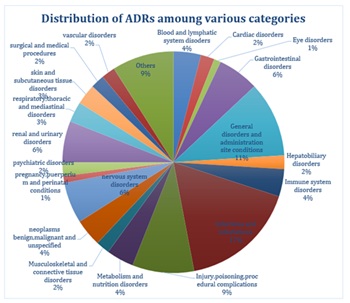
Figure 2: Distribution of Adverse Drug Reactions of Tacrolimus.
In this case, patient developed hyperpigmentation
following the use of topical tacrolimus. Since the pigmentation in the patient
resolved after tacrolimus withdrawal, there is a probable definite causal
relationship between the drug and the ADR. Hence, this case is worth reporting.
4.
Conclusion
This particular case illustrates hyperpigmentation; an
uncommon but significant ADR linked to topical tacrolimus treatment. Clinicians
should be on the lookout for rare dermatological adverse effects like DIP, even
though tacrolimus is frequently used to treat inflammatory skin disorders like
atopic dermatitis. To avoid long-term cosmetic issues, early detection, timely
removal of the offending substance and patient counselling are crucial. Since
this ADR is uncommon, recording and disclosing such cases helps to improve
pharmacovigilance and gain a better knowledge of the side effects associated
with tacrolimus.
5.
References
3.
Tisack A,
Mohammad TF. Drug-Induced Pigmentation: A Review. Drugs, 2024;84: 1071-1091.
6.
Araya AA,
Tasnif Y. Tacrolimus. In: StatPearls. Treasure Island (FL): StatPearls
Publishing, 2025.
7.
VigiAccess.
World Health Organisation, 2015.